Development and Characterization of Sustainable Composites from Bacterial Polyester Poly(3-Hydroxybutyrate-co-3-hydroxyhexanoate) and Almond Shell Flour by Reactive Extrusion with Oligomers of Lactic Acid
Abstract
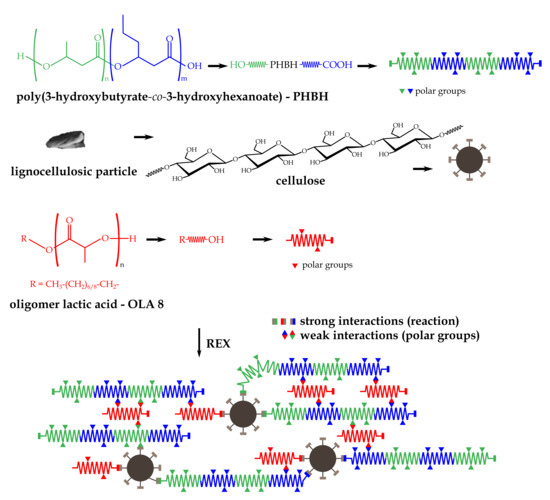
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Manufacturing of PHBH-ASF Composites
2.3. Mechanical Properties of PHBH-ASF/OLA Composites
2.4. Morphology of PHBH-ASF/OLA Composites
2.5. Thermal Characterization of PHBH-ASF/OLA Composites
2.6. Thermomechanical Characterization of PHBH-ASF/OLA Composites
2.7. Water Uptake of PHBH-ASF/OLA Composites
3. Results and Discussion
3.1. Mechanical Properties of PHBH-ASF/OLA Composites
3.2. Thermal Properties of PHBH-ASF/OLA Composites
3.3. Thermomechanical Properties of PHBH-ASF/OLA Composites
3.4. Evolution of the Water Uptake and Water Diffusion Process in PHBH-ASF/OLA Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carbonell-Verdu, A.; Bernardi, L.; Garcia-Garcia, D.; Sanchez-Nacher, L.; Balart, R. Development of environmentally friendly composite matrices from epoxidized cottonseed oil. Eur. Polym. J. 2015, 63, 1–10. [Google Scholar] [CrossRef]
- España, J.; Samper, M.; Fages, E.; Sánchez-Nácher, L.; Balart, R. Investigation of the effect of different silane coupling agents on mechanical performance of basalt fiber composite laminates with biobased epoxy matrices. Polym. Compos. 2013, 34, 376–381. [Google Scholar] [CrossRef]
- Basalp, D.; Tihminlioglu, F.; Sofuoglu, S.C.; Inal, F.; Sofuoglu, A. Utilization of Municipal Plastic and Wood Waste in Industrial Manufacturing of Wood Plastic Composites. Waste Biomass Valorization 2020. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Mohanty, A.K. Wood fiber reinforced bacterial bioplastic composites: Fabrication and performance evaluation. Compos. Sci. Technol. 2007, 67, 1753–1763. [Google Scholar] [CrossRef]
- Mukheem, A.; Hossain, M.; Shahabuddin, S.; Muthoosamy, K.; Manickam, S.; Sudesh, K.; Saidur, R.; Sridewi, N.; Campus, N.M. Bioplastic Polyhydroxyalkanoate (PHA): Recent Advances in Modification and Medical Applications. Prepr. Org. 2018. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Sustainable bio-composites from renewable resources: Opportunities and challenges in the green materials world. Abstr. Pap. Am. Chem. Soc. 2002, 223, D70. [Google Scholar]
- Petchwattana, N.; Covavisaruch, S. Mechanical and Morphological Properties of Wood Plastic Biocomposites Prepared from Toughened Poly(lactic acid) and Rubber Wood Sawdust (Hevea brasiliensis). J. Bionic Eng. 2014, 11, 630–637. [Google Scholar] [CrossRef]
- Summerscales, J.; Dissanayake, N.; Virk, A.; Hall, W. A review of bast fibres and their composites. Part 2-Composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1336–1344. [Google Scholar] [CrossRef] [Green Version]
- Averous, L. Biodegradable multiphase systems based on plasticized starch: A review. J. Macromol. Sci. Polym. Rev. 2004, C44, 231–274. [Google Scholar] [CrossRef]
- Yang, Y.; Ke, S.; Ren, L.; Wang, Y.; Li, Y.; Huang, H. Dielectric spectroscopy of biodegradable poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) films. Eur. Polym. J. 2012, 48, 79–85. [Google Scholar] [CrossRef]
- Liao, Q.; Noda, I.; Frank, C.W. Melt viscoelasticity of biodegradable poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) copolymers. Polymer 2009, 50, 6139–6148. [Google Scholar] [CrossRef]
- Alata, H.; Aoyama, T.; Inoue, Y. Effect of aging on the mechanical properties of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Macromolecules 2007, 40, 4546–4551. [Google Scholar] [CrossRef]
- Misra, S.K.; Valappil, S.P.; Roy, I.; Boccaccini, A.R. Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules 2006, 7, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, H.; Pegoretti, A.; Brusa, R.S.; Ceccato, R.; Penasa, L.; Tarter, S.; Checchetto, R. Molecular transport through 3-hydroxybutyrate co-3-hydroxyhexanoate biopolymer films with dispersed graphene oxide nanoparticles: Gas barrier, structural and mechanical properties. Polym. Test. 2019, 81, 106181. [Google Scholar] [CrossRef]
- Corre, Y.-M.; Bruzaud, S.; Audic, J.-L.; Grohens, Y. Morphology and functional properties of commercial polyhydroxyalkanoates: A comprehensive and comparative study. Polym. Test. 2012, 31, 226–235. [Google Scholar] [CrossRef]
- Watanabe, T.; He, Y.; Fukuchi, T.; Inoue, Y. Comonomer compositional distribution and thermal characteristics of bacterially synthesized poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)s. Macromol. Biosci. 2001, 1, 75–83. [Google Scholar] [CrossRef]
- Oyama, T.; Kobayashi, S.; Okura, T.; Sato, S.; Tajima, K.; Isono, T.; Satoh, T. Biodegradable Compatibilizers for Poly(hydroxyalkanoate)/Poly(epsilon-caprolactone) Blends through Click Reactions with End-Functionalized Microbial Poly(hydroxyalkanoate)s. ACS Sustain. Chem. Eng. 2019, 7, 7969. [Google Scholar] [CrossRef]
- Sato, H.; Nakamura, M.; Padermshoke, A.; Yamaguchi, H.; Terauchi, H.; Ekgasit, S.; Noda, I.; Ozaki, Y. Thermal behavior and molecular interaction of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) studied by wide-angle X-ray diffraction. Macromolecules 2004, 37, 3763–3769. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Sato, H.; Noda, I.; Ozaki, Y. Multiple melting behavior of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) investigated by differential scanning calorimetry and infrared spectroscopy. Polymer 2007, 48, 4777–4785. [Google Scholar] [CrossRef]
- Xu, P.; Cao, Y.; Lv, P.; Ma, P.; Dong, W.; Bai, H.; Wang, W.; Du, M.; Chen, M. Enhanced crystallization kinetics of bacterially synthesized poly(3-hydroxybutyrate-co-3-hydroxyhexanate) with structural optimization of oxalamide compounds as nucleators. Polym. Degrad. Stab. 2018, 154, 170–176. [Google Scholar] [CrossRef]
- Tham, W.L.; Ishak, Z.A.M.; Chow, W.S. Water Absorption and Hygrothermal Aging Behaviors of SEBS-g-MAH Toughened Poly(lactic acid)/Halloysite Nanocomposites. Polym. Plast. Technol. Eng. 2014, 53, 472–480. [Google Scholar] [CrossRef]
- Tham, W.L.; Poh, B.T.; Ishak, Z.A.M.; Chow, W.S. Water Absorption Kinetics and Hygrothermal Aging of Poly(lactic acid) Containing Halloysite Nanoclay and Maleated Rubber. J. Polym. Environ. 2015, 23, 242–250. [Google Scholar] [CrossRef]
- Arbelaiz, A.; Fernandez, B.; Ramos, J.A.; Retegi, A.; Llano-Ponte, R.; Mondragon, I. Mechanical properties of short flax fibre bundle/polypropylene composites: Influence of matrix/fibre modification, fibre content, water uptake and recycling. Compos. Sci. Technol. 2005, 65, 1582–1592. [Google Scholar] [CrossRef]
- Deroine, M.; Le Duigou, A.; Corre, Y.-M.; Le Gac, P.-Y.; Davies, P.; Cesar, G.; Bruzaud, S. Accelerated ageing of polylactide in aqueous environments: Comparative study between distilled water and seawater. Polym. Degrad. Stab. 2014, 108, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Gil-Castell, O.; Badia, J.D.; Kittikorn, T.; Stromberg, E.; Martinez-Felipe, A.; Ek, M.; Karlsson, S.; Ribes-Greus, A. Hydrothermal ageing of polylactide/sisal biocomposites. Studies of water absorption behaviour and Physico-Chemical performance. Polym. Degrad. Stab. 2014, 108, 212–222. [Google Scholar] [CrossRef]
- Petinakis, E.; Yu, L.; Edward, G.; Dean, K.; Liu, H.; Scully, A.D. Effect of Matrix-Particle Interfacial Adhesion on the Mechanical Properties of Poly(lactic acid)/Wood-Flour Micro-Composites. J. Polym. Environ. 2009, 17, 83–94. [Google Scholar] [CrossRef]
- Pilla, S.; Gong, S.; O’Neill, E.; Rowell, R.M.; Krzysik, A.M. Polylactide-pine wood flour composites. Polym. Eng. Sci. 2008, 48, 578–587. [Google Scholar] [CrossRef]
- Shah, B.L.; Selke, S.E.; Walters, M.B.; Heiden, P.A. Effects of wood flour and chitosan on mechanical, chemical, and thermal properties of polylactide. Polym. Compos. 2008, 29, 655–663. [Google Scholar] [CrossRef]
- Balart, J.F.; Garcia-Sanoguera, D.; Balart, R.; Boronat, T.; Sanchez-Nacher, L. Manufacturing and properties of biobased thermoplastic composites from poly(lactid acid) and hazelnut shell wastes. Polym. Compos. 2018, 39, 848–857. [Google Scholar] [CrossRef]
- Kumar, S.; Vedrtnam, A.; Pawar, S.J. Effect of wood dust type on mechanical properties, wear behavior, biodegradability, and resistance to natural weathering of wood-plastic composites. Front. Struct. Civ. Eng. 2019, 13, 1446–1462. [Google Scholar] [CrossRef]
- Ling, S.L.; Koay, S.C.; Chan, M.Y.; Tshai, K.Y.; Chantara, T.R.; Pang, M.M. Wood Plastic Composites Produced from Postconsumer Recycled Polystyrene and Coconut Shell: Effect of Coupling Agent and Processing Aid on Tensile, Thermal, and Morphological Properties. Polym. Eng. Sci. 2020, 60, 202–210. [Google Scholar] [CrossRef]
- Quitadamo, A.; Massardier, V.; Valente, M. Eco-Friendly Approach and Potential Biodegradable Polymer Matrix for WPC Composite Materials in Outdoor Application. Int. J. Polym. Sci. 2019. [Google Scholar] [CrossRef]
- Salasinska, K.; Polka, M.; Gloc, M.; Ryszkowska, J. Natural fiber composites: The effect of the kind and content of filler on the dimensional and fire stability of polyolefin-based composites. Polimery 2016, 61, 255–265. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Z.; McDonald, A.G. Effect of Different Reinforcing Fillers on Properties, Interfacial Compatibility and Weatherability of Wood-plastic Composites. J. Bionic Eng. 2019, 16, 337–353. [Google Scholar] [CrossRef]
- Yussuf, A.A.; Massoumi, I.; Hassan, A. Comparison of Polylactic Acid/Kenaf and Polylactic Acid/Rise Husk Composites: The Influence of the Natural Fibers on the Mechanical, Thermal and Biodegradability Properties. J. Polym. Environ. 2010, 18, 422–429. [Google Scholar] [CrossRef]
- Kuciel, S.; Jakubowska, P.; Kuzniar, P. A study on the mechanical properties and the influence of water uptake and temperature on biocomposites based on polyethylene from renewable sources. Compos. Part B-Eng. 2014, 64, 72–77. [Google Scholar] [CrossRef]
- Liminana, P.; Quiles-Carrillo, L.; Boronat, T.; Balart, R.; Montanes, N. The Effect of Varying Almond Shell Flour (ASF) Loading in Composites with Poly(Butylene Succinate (PBS) Matrix Compatibilized with Maleinized Linseed Oil (MLO). Materials 2018, 11, 2179. [Google Scholar] [CrossRef] [Green Version]
- Quiles-Carrillo, L.; Montanes, N.; Garcia-Garcia, D.; Carbonell-Verdu, A.; Balart, R.; Torres-Giner, S. Effect of different compatibilizers on injection-molded green composite pieces based on polylactide filled with almond shell flour. Compos. Part B Eng. 2018, 147, 76–85. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Sain, M. Mechanical properties of biodegradable composites from poly lactic acid (PLA) and microcrystalline cellulose (MCC). J. Appl. Polym. Sci. 2005, 97, 2014–2025. [Google Scholar] [CrossRef]
- Ghaffar, S.H.; Madyan, O.A.; Fan, M.; Corker, J. The Influence of Additives on the Interfacial Bonding Mechanisms between Natural Fibre and Biopolymer Composites. Macromol. Res. 2018, 26, 851–863. [Google Scholar] [CrossRef]
- Tserki, V.; Matzinos, P.; Kokkou, S.; Panayiotou, C. Novel biodegradable composites based on treated lignocellulosic waste flour as filler. Part I. Surface chemical modification and characterization of waste flour. Compos. Part A Appl. Sci. Manuf. 2005, 36, 965–974. [Google Scholar] [CrossRef]
- Niaraki, P.R.; Krause, A. Correlation between physical bonding and mechanical properties of wood plastic composites: Part 1: Interaction of chemical and mechanical treatments on physical properties. J. Adhes. Sci. Technol. 2020, 34, 744–755. [Google Scholar] [CrossRef]
- Akesson, D.; Fazelinejad, S.; Skrifvars, V.-V.; Skrifvars, M. Mechanical recycling of polylactic acid composites reinforced with wood fibres by multiple extrusion and hydrothermal ageing. J. Reinf. Plast. Compos. 2016, 35, 1248–1259. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Montanes, N.; Fenollar, O.; García-Sanoguera, D.; Balart, R. Development and optimization of renewable vinyl plastisol/wood flour composites exposed to ultraviolet radiation. Mater. Des. 2016, 108, 648–658. [Google Scholar] [CrossRef]
- Juárez, D.; Ferrand, S.; Fenollar, O.; Fombuena, V.; Balart, R. Improvement of thermal inertia of styrene–ethylene/butylene–styrene (SEBS) polymers by addition of microencapsulated phase change materials (PCMs). Eur. Polym. J. 2011, 47, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Pracella, M.; Haque, M.; Alvarez, V. Functionalization, compatibilization and properties of polyolefin composites with natural fibers. Polymers 2010, 2, 554–574. [Google Scholar] [CrossRef] [Green Version]
- Chabros, A.; Gawdzik, B.; Podkościelna, B.; Goliszek, M.; Pączkowski, P. Composites of Unsaturated Polyester Resins with Microcrystalline Cellulose and Its Derivatives. Materials 2020, 13, 62. [Google Scholar] [CrossRef] [Green Version]
- Mokhena, T.; Sefadi, J.; Sadiku, E.; John, M.; Mochane, M.; Mtibe, A. Thermoplastic processing of PLA/cellulose nanomaterials composites. Polymers 2018, 10, 1363. [Google Scholar] [CrossRef] [Green Version]
- Patwa, R.; Saha, N.; Saha, P.; Katiyar, V. Biocomposites of poly(lactic acid) and lactic acid oligomer-grafted bacterial cellulose: It’s preparation and characterization. J. Appl. Polym. Sci. 2019, 136. [Google Scholar] [CrossRef]
- Tripathi, N.; Katiyar, V. Lactic acid oligomer (OLLA) grafted gum arabic based green adhesive for structural applications. Int. J. Biol. Macromol. 2018, 120, 711–720. [Google Scholar] [CrossRef]
- Lascano, D.; Moraga, G.; Ivorra-Martinez, J.; Rojas-Lema, S.; Torres-Giner, S.; Balart, R.; Boronat, T.; Quiles-Carrillo, L. Development of Injection-Molded Polylactide Pieces with High Toughness by the Addition of Lactic Acid Oligomer and Characterization of Their Shape Memory Behavior. Polymers 2019, 11, 2099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.-x.; Huang, Z.-g.; Diao, X.-q.; Weng, Y.-x.; Wang, Y.-Z. Characterization of the effect of REC on the compatibility of PHBH and PLA. Polym. Test. 2015, 42, 17–25. [Google Scholar] [CrossRef]
- Asrar, J.; Valentin, H.E.; Berger, P.A.; Tran, M.; Padgette, S.R.; Garbow, J.R. Blosynthesis and properties of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) polymers. Biomacromolecules 2002, 3, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Cheng, B.; Wu, Q. DSC analysis of isothermally melt-crystallized bacterial poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) films. J. Therm. Anal. Calorim. 2011, 103, 1001–1006. [Google Scholar] [CrossRef]
- Jacquel, N.; Tajima, K.; Nakamura, N.; Miyagawa, T.; Pan, P.; Inoue, Y. Effect of Orotic Acid as a Nucleating Agent on the Crystallization of Bacterial Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Copolymers. J. Appl. Polym. Sci. 2009, 114, 1287–1294. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Duart, S.; Montanes, N.; Torres-Giner, S.; Balart, R. Enhancement of the mechanical and thermal properties of injection-molded polylactide parts by the addition of acrylated epoxidized soybean oil. Mater. Des. 2018, 140, 54–63. [Google Scholar] [CrossRef]
- Burgos, N.; Martino, V.P.; Jiménez, A. Characterization and ageing study of poly (lactic acid) films plasticized with oligomeric lactic acid. Polym. Degrad. Stab. 2013, 98, 651–658. [Google Scholar] [CrossRef]
- Armentano, I.; Fortunati, E.; Burgos, N.; Dominici, F.; Luzi, F.; Fiori, S.; Jiménez, A.; Yoon, K.; Ahn, J.; Kang, S. Processing and characterization of plasticized PLA/PHB blends for biodegradable multiphase systems. Expresss Polym. Lett. 2015, 9, 583–596. [Google Scholar] [CrossRef]
- Miquelard, G.; Guinault, A.; Sollogoub, C.; Gervais, M. Combined compatibilization and plasticization effect of low molecular weight poly (lactic acid) in poly (lactic acid)/poly (3-hydroxybutyrate-co-3-hydroxyvalerate) blends. Expresss Polym. Lett. 2018, 12, 114–125. [Google Scholar] [CrossRef]
- Ferri, J.M.; Garcia-Garcia, D.; Montanes, N.; Fenollar, O.; Balart, R. The effect of maleinized linseed oil as biobased plasticizer in poly (lactic acid)-based formulations. Polym. Int. 2017, 66, 882–891. [Google Scholar] [CrossRef]
- Garcia-Campo, M.J.; Quiles-Carrillo, L.; Masia, J.; Reig-Pérez, M.J.; Montanes, N.; Balart, R. Environmentally friendly compatibilizers from soybean oil for ternary blends of poly (lactic acid)-PLA, poly (ε-caprolactone)-PCL and poly (3-hydroxybutyrate)-PHB. Materials 2017, 10, 1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liminana, P.; Garcia-Sanoguera, D.; Quiles-Carrillo, L.; Balart, R.; Montanes, N. Development and characterization of environmentally friendly composites from poly (butylene succinate)(PBS) and almond shell flour with different compatibilizers. Compos. Part B Eng. 2018, 144, 153–162. [Google Scholar] [CrossRef]
- Thomas, S.; Shumilova, A.; Kiselev, E.; Baranovsky, S.; Vasiliev, A.; Nemtsev, I.; Kuzmin, A.P.; Sukovatyi, A.; Avinash, R.P.; Volova, T. Thermal, mechanical and biodegradation studies of biofiller based poly-3-hydroxybutyrate biocomposites. Int. J. Biol. Macromol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Gao, X.; Tang, C.Y.; Law, W.C.; Chen, L.; Hu, T.; Wu, C.; Tsui, C.P.; Rao, N. Compatibilization of poly (lactic acid)/high impact polystyrene interface using copolymer poly (stylene-ran-methyl acrylate). J. Appl. Polym. Sci. 2018, 135, 45799. [Google Scholar] [CrossRef]
- Hosoda, N.; Tsujimoto, T.; Uyama, H. Green Composite of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Reinforced with Porous Cellulose. Acs Sustain. Chem. Eng. 2014, 2, 248–253. [Google Scholar] [CrossRef]
- Perinovic, S.; Andricic, B.; Erceg, M. Thermal properties of poly(L-lactide)/olive stone flour composites. Thermochim. Acta 2010, 510, 97–102. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Sammon, C.; Balart, R.; Torres-Giner, S. Compatibilization of highly sustainable polylactide/almond shell flour composites by reactive extrusion with maleinized linseed oil. Ind. Crop. Prod. 2018, 111, 878–888. [Google Scholar] [CrossRef]
- Liminana, P.; Garcia-Sanoguera, D.; Quiles-Carrillo, L.; Balart, R.; Montanes, N. Optimization of Maleinized Linseed Oil Loading as a Biobased Compatibilizer in Poly(Butylene Succinate) Composites with Almond Shell Flour. Materials 2019, 12, 685. [Google Scholar] [CrossRef] [Green Version]
- Yin, C.; Wang, Z.; Luo, Y.; Li, J.; Zhou, Y.; Zhang, X.; Zhang, H.; Fang, P.; He, C. Thermal annealing on free volumes, crystallinity and proton conductivity of Nafion membranes. J. Phys. Chem. Solids 2018, 120, 71–78. [Google Scholar] [CrossRef]
- Oliver-Ortega, H.; Méndez, J.A.; Espinach, F.X.; Tarrés, Q.; Ardanuy, M.; Mutjé, P. Impact strength and water uptake behaviors of fully bio-based PA11-SGW composites. Polymers 2018, 10, 717. [Google Scholar] [CrossRef] [Green Version]
- Pfister, D.P.; Larock, R.C. Thermophysical properties of conjugated soybean oil/corn stover biocomposites. Bioresour. Technol. 2010, 101, 6200–6206. [Google Scholar] [CrossRef] [PubMed]






| Code | PHBH (wt %) | ASF (wt %) | OLA (phr) |
|---|---|---|---|
| PHBH | 100 | - | - |
| PHBH-10ASF | 90 | 10 | - |
| PHBH-20ASF | 80 | 20 | - |
| PHBH-30ASF | 70 | 30 | - |
| PHBH-30ASF/10OLA | 70 | 30 | 10 |
| PHBH-30ASF/20OLA | 70 | 30 | 20 |
| Code | Tensile Strength (MPa) | Elastic Modulus (MPa) | Elongation at Break (%) | Hardness Shore-D | Impact Strength (kJ m−2) |
|---|---|---|---|---|---|
| PHBH | 20 ± 1 | 1065 ± 23 | 8.1 ± 0.7 | 60.2 ± 0.2 | 4.3 ± 0.3 |
| PHBH-10ASF | 16 ± 1 | 1310 ± 35 | 5.2 ± 0.4 | 63.5 ± 0.4 | 1.8 ± 0.2 |
| PHBH-20ASF | 14 ± 1 | 1543 ± 23 | 4.0 ± 0.4 | 64.7 ± 0.6 | 1.7 ± 0.2 |
| PHBH-30ASF | 12 ± 1 | 1744 ± 31 | 3.5 ± 0.3 | 66.2 ± 0.6 | 1.6 ± 0.3 |
| PHBH-30ASF/10OLA | 10 ± 1 | 1158 ± 23 | 6.2 ± 0.2 | 58.6 ± 0.5 | 2.4 ± 0.4 |
| PHBH-30ASF/20OLA | 8 ± 1 | 735 ± 28 | 9.7 ± 0.8 | 50.0 ± 0.4 | 2.9 ± 0.3 |
| Code | Tg (°C) | Tcc (°C) | Tm1 (°C) | Tm2 (°C) | Tm3 (°C) | ΔHm1* (J g−1) | ΔHcc2 (J g−1) | ΔHm2 (J g−1) | Xc1* (%) | Xc2 (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| PHBH | 0.3 ± 0.1 | 54.6 ± 1.1 | 111.5 ± 1.9 | 130.8 ± 2.0 | 162.5 ± 1.2 | 20.3 ± 0.5 | 26.7 ± 0.8 | 33.6 ± 1.3 | 13.9 ± 1.1 | 4.7 ± 0.3 |
| PHBH-10ASF | −0.5 ± 0.1 | 57.5 ± 1.8 | 112.1 ± 1.8 | 129.7 ± 1.7 | 162.3 ± 1.8 | 19.6 ± 0.4 | 17.4 ± 0.4 | 29.0 ± 2.2 | 14.9 ± 1.1 | 8.8 ± 0.4 |
| PHBH-20ASF | −1.9 ± 0.2 | 55.5 ± 1.9 | 111.5 ± 2.0 | 129.6 ± 2.1 | 163.4 ± 1.4 | 18.1 ± 0.3 | 15.0 ± 0.1 | 26.4 ± 2.1 | 15.5 ± 0.8 | 9.8 ± 0.7 |
| PHBH-30ASF | −1.1 ± 0.1 | 51.8 ± 1.2 | 111.3 ± 1.3 | 130.6 ± 1.9 | 164.1 ± 1.2 | 8.5 ± 0.2 | 3.7 ± 0.3 | 21.4 ± 0.9 | 8.3 ± 0.7 | 17.3 ± 0.9 |
| PHBH-30ASF/10OLA | −5.2 ± 0.2 | 56.3 ± 1.4 | 107.6 ± 2.1 | 127.0 ± 1.8 | 159.4 ± 1.6 | 7.4 ± 0.1 | 11.4 ± 0.1 | 26.1 ± 1.3 | 8.0 ± 0.8 | 14.7 ± 0.8 |
| PHBH-30ASF/20OLA | −5.6 ± 0.3 | 62.6 ± 2.0 | 109.9 ± 2.3 | 127.5 ± 2.0 | 157.9 ± 1.5 | 6.4 ± 0.3 | 15.9 ± 0.4 | 22.6 ± 1.5 | 7.5 ± 1.2 | 6.7 ± 0.4 |
| Code | T2% (°C) | Tdeg (°C) | Residual Mass (wt %) |
|---|---|---|---|
| ASF | 101.4 * | 300.6/460.7 | 1.5 ± 0.2 |
| PHBH | 286.8 | 308.9 | 2.4 ± 0.3 |
| PHBH-10ASF | 253.2 | 288.1 | 2.3 ± 0.2 |
| PHBH-20ASF | 250.5 | 284.3 | 2.1 ± 0.1 |
| PHBH-30ASF | 223.6 | 279.1 | 2.0 ± 0.3 |
| PHBH-30ASF/10OLA | 258.4 | 292.0 | 2.0 ± 0.2 |
| PHBH-30ASF/20OLA | 226.3 | 283.5 | 2.0 ± 0.2 |
| Code | Tg (°C) | E’ at −40 °C (MPa) | E’ at 25 °C (MPa) |
|---|---|---|---|
| PHBH | 10.6 ± 0.9 | 1869 ± 42 | 1345 ± 28 |
| PHBH-10ASF | 14.3 ± 0.8 | 1910 ± 49 | 1431 ± 40 |
| PHBH-20ASF | 12.0 ± 0.7 | 1948 ± 30 | 1512 ± 20 |
| PHBH-30ASF | 11.4 ± 0.9 | 2019 ± 52 | 1604 ± 45 |
| PHBH-30ASF/10OLA | 9.7 ± 0.7 | 1601 ± 36 | 1352 ± 29 |
| PHBH-30ASF/10OLA | 9.3 ± 0.6 | 853 ± 25 | 767 ± 23 |
| Code | Tg (°C) | CLTE (μm m−1 °C−1) | |
|---|---|---|---|
| Below Tg | Above Tg | ||
| PHBH | −0.3 ± 0.1 | 77.1 ± 2.2 | 160.7 ± 2.3 |
| PHBH-10ASF | 0.2 ± 0.1 | 76.9 ± 2.1 | 157.0 ± 1.3 |
| PHBH-20ASF | −0.4 ± 0.1 | 75.6 ± 2.1 | 157.4 ± 2.9 |
| PHBH-30ASF | 1.4 ± 0.2 | 66.8 ± 0.8 | 140.3 ± 2.6 |
| PHBH-30ASF/10OLA | −1.3 ± 0.1 | 72.0 ± 0.9 | 169.1 ± 3.8 |
| PHBH-30ASF/20OLA | −1.4 ± 0.2 | 90.7 ± 4.1 | 194.3 ± 2.83 |
| Code | D × 10−9 (cm2 s−1) | Dc × 10−9 (cm2 s−1) |
|---|---|---|
| PHBH | 0.14 ± 0.03 | 0.07 ± 0.01 |
| PHBH-10ASF | 0.54 ± 0.05 | 0.25 ± 0.02 |
| PHBH-20ASF | 1.56 ± 0.07 | 0.74 ± 0.04 |
| PHBH-30ASF | 6.08 ± 0.08 | 2.89 ± 0.05 |
| PHBH-30ASF/10OLA | 6.66 ± 0.09 | 3.17 ± 0.07 |
| PHBH-30ASF/20OLA | 7.08 ± 0.09 | 3.37 ± 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivorra-Martinez, J.; Manuel-Mañogil, J.; Boronat, T.; Sanchez-Nacher, L.; Balart, R.; Quiles-Carrillo, L. Development and Characterization of Sustainable Composites from Bacterial Polyester Poly(3-Hydroxybutyrate-co-3-hydroxyhexanoate) and Almond Shell Flour by Reactive Extrusion with Oligomers of Lactic Acid. Polymers 2020, 12, 1097. https://doi.org/10.3390/polym12051097
Ivorra-Martinez J, Manuel-Mañogil J, Boronat T, Sanchez-Nacher L, Balart R, Quiles-Carrillo L. Development and Characterization of Sustainable Composites from Bacterial Polyester Poly(3-Hydroxybutyrate-co-3-hydroxyhexanoate) and Almond Shell Flour by Reactive Extrusion with Oligomers of Lactic Acid. Polymers. 2020; 12(5):1097. https://doi.org/10.3390/polym12051097
Chicago/Turabian StyleIvorra-Martinez, Juan, Jose Manuel-Mañogil, Teodomiro Boronat, Lourdes Sanchez-Nacher, Rafael Balart, and Luis Quiles-Carrillo. 2020. "Development and Characterization of Sustainable Composites from Bacterial Polyester Poly(3-Hydroxybutyrate-co-3-hydroxyhexanoate) and Almond Shell Flour by Reactive Extrusion with Oligomers of Lactic Acid" Polymers 12, no. 5: 1097. https://doi.org/10.3390/polym12051097
APA StyleIvorra-Martinez, J., Manuel-Mañogil, J., Boronat, T., Sanchez-Nacher, L., Balart, R., & Quiles-Carrillo, L. (2020). Development and Characterization of Sustainable Composites from Bacterial Polyester Poly(3-Hydroxybutyrate-co-3-hydroxyhexanoate) and Almond Shell Flour by Reactive Extrusion with Oligomers of Lactic Acid. Polymers, 12(5), 1097. https://doi.org/10.3390/polym12051097