Manufacturing and Properties of Binary Blend from Bacterial Polyester Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) and Poly(caprolactone) with Improved Toughness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Manufacturing of PHBH/PCL Binary Blends
2.3. Characterizations Techniques
2.3.1. Thermal and Thermomechanical Characterization
2.3.2. Mechanical Properties
2.3.3. Morphology Characterization
3. Results and Discussion
3.1. Thermal Properties of PHBH/PCL Blends
3.2. Thermomechanical Properties of PHBH/PCL Blends
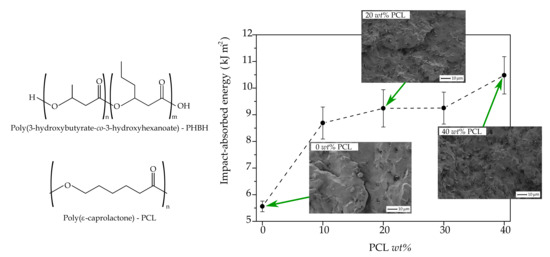
3.3. Mechanical Properties and Morphology of PHBH/PCL Blends
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fombuena, V.; Samper, M.D. Study of the properties of thermoset materials derived from epoxidized soybean oil and protein fillers. J. Am. Oil Chem. Soc. 2013, 90, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Carbonell-Verdu, A.; Bernardi, L.; Garcia-Garcia, D.; Sanchez-Nacher, L.; Balart, R. Development of environmentally friendly composite matrices from epoxidized cottonseed oil. Eur. Polym. J. 2015, 63, 1–10. [Google Scholar] [CrossRef]
- Ferrero, B.; Boronat, T.; Moriana, R.; Fenollar, O.; Balart, R. Green composites based on wheat gluten matrix and posidonia oceanica waste fibers as reinforcements. Polym. Compos. 2013, 34, 1663–1669. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Sammon, C.; Balart, R.; Torres-Giner, S. Compatibilization of highly sustainable polylactide/almond shell flour composites by reactive extrusion with maleinized linseed oil. Ind. Crop. Prod. 2018, 111, 878–888. [Google Scholar] [CrossRef]
- Wong, J.X.; Ogura, K.; Chen, S.; Rehm, B.H. Bioengineered Polyhydroxyalkanoates as Immobilized Enzyme Scaffolds for Industrial Applications. Front. Bioeng. Biotechnol. 2020, 8, 156. [Google Scholar] [CrossRef] [Green Version]
- Dang, K.M.; Yoksan, R. Development of thermoplastic starch blown film by incorporating plasticized chitosan. Carbohydr. Polym. 2015, 115, 575–581. [Google Scholar] [CrossRef]
- Carter, P.; Rahman, S.M.; Bhattarai, N. Facile fabrication of aloe vera containing PCL nanofibers for barrier membrane application. J. Biomater. Sci. Polym. Ed. 2016, 27, 692–708. [Google Scholar] [CrossRef]
- Zhang, W.; Xiang, Y.; Fan, H.; Wang, L.; Xie, Y.; Zhao, G.; Liu, Y. Biodegradable urea-formaldehyde/PBS and its ternary nanocomposite prepared by a novel and scalable reactive extrusion process for slow-release applications in agriculture. J. Agric. Food Chem. 2020, 68, 4595–4606. [Google Scholar] [CrossRef]
- Boronat, T.; Fombuena, V.; Garcia-Sanoguera, D.; Sanchez-Nacher, L.; Balart, R. Development of a biocomposite based on green polyethylene biopolymer and eggshell. Mater. Des. 2015, 68, 177–185. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López, J. On the use of PLA-PHB blends for sustainable food packaging applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef]
- Mukherjee, T.; Kao, N. PLA based biopolymer reinforced with natural fibre: A review. J. Polym. Environ. 2011, 19, 714–725. [Google Scholar] [CrossRef]
- Averous, L. Biodegradable multiphase systems based on plasticized starch: A review. J. Macromol. Sci. Part C 2004, 44, 231–274. [Google Scholar] [CrossRef]
- Pilania, G.; Iverson, C.N.; Lookman, T.; Marrone, B.L. Machine-Learning-Based Predictive Modeling of Glass Transition Temperatures: A Case of Polyhydroxyalkanoate Homopolymers and Copolymers. J. Chem. Inf. Model. 2019, 59, 5013–5025. [Google Scholar] [CrossRef] [PubMed]
- Asrar, J.; Valentin, H.E.; Berger, P.A.; Tran, M.; Padgette, S.R.; Garbow, J.R. Biosynthesis and properties of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) polymers. Biomacromolecules 2002, 3, 1006–1012. [Google Scholar] [CrossRef]
- Misra, S.K.; Valappil, S.P.; Roy, I.; Boccaccini, A.R. Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules 2006, 7, 2249–2258. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Montanes, N.; Boronat, T.; Quiles-Carrillo, L.; Balart, R. Melt grafting of sepiolite nanoclay onto poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by reactive extrusion with multi-functional epoxy-based styrene-acrylic oligomer. Eur. Polym. J. 2016, 84, 693–707. [Google Scholar] [CrossRef]
- Corre, Y.-M.; Bruzaud, S.; Audic, J.-L.; Grohens, Y. Morphology and functional properties of commercial polyhydroxyalkanoates: A comprehensive and comparative study. Polym. Test. 2012, 31, 226–235. [Google Scholar] [CrossRef]
- Alata, H.; Aoyama, T.; Inoue, Y. Effect of aging on the mechanical properties of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Macromolecules 2007, 40, 4546–4551. [Google Scholar] [CrossRef]
- Mahmood, H.; Pegoretti, A.; Brusa, R.S.; Ceccato, R.; Penasa, L.; Tarter, S.; Checchetto, R. Molecular transport through 3-hydroxybutyrate co-3-hydroxyhexanoate biopolymer films with dispersed graphene oxide nanoparticles: Gas barrier, structural and mechanical properties. Polym. Test. 2020, 81, 106181. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Fenollar, O.; Fombuena, V.; Lopez-Martinez, J.; Balart, R. Improvement of Mechanical Ductile Properties of Poly(3-hydroxybutyrate) by Using Vegetable Oil Derivatives. Macromol. Mater. Eng. 2017, 302, 1600330. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Sato, H.; Noda, I.; Ozaki, Y. Multiple melting behavior of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) investigated by differential scanning calorimetry and infrared spectroscopy. Polymer 2007, 48, 4777–4785. [Google Scholar] [CrossRef]
- Sharma, P.K.; Munir, R.I.; Blunt, W.; Dartiailh, C.; Cheng, J.; Charles, T.C.; Levin, D.B. Synthesis and physical properties of polyhydroxyalkanoate polymers with different monomer compositions by recombinant Pseudomonas putida LS46 expressing a novel PHA synthase (PhaC116) enzyme. Appl. Sci. 2017, 7, 242. [Google Scholar] [CrossRef]
- Watanabe, T.; He, Y.; Fukuchi, T.; Inoue, Y. Comonomer compositional distribution and thermal characteristics of bacterially synthesized poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) s. Macromol. Biosci. 2001, 1, 75–83. [Google Scholar] [CrossRef]
- Morgan-Sagastume, F.; Valentino, F.; Hjort, M.; Cirne, D.; Karabegovic, L.; Gerardin, F.; Johansson, P.; Karlsson, A.; Magnusson, P.; Alexandersson, T. Polyhydroxyalkanoate (PHA) production from sludge and municipal wastewater treatment. Water Sci. Technol. 2014, 69, 177–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, H.; Nakamura, M.; Padermshoke, A.; Yamaguchi, H.; Terauchi, H.; Ekgasit, S.; Noda, I.; Ozaki, Y. Thermal behavior and molecular interaction of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) studied by wide-angle X-ray diffraction. Macromolecules 2004, 37, 3763–3769. [Google Scholar] [CrossRef]
- Oyama, T.; Kobayashi, S.; Okura, T.; Sato, S.; Tajima, K.; Isono, T.; Satoh, T. Biodegradable Compatibilizers for Poly(hydroxyalkanoate)/Poly(ε-caprolactone) Blends through Click Reactions with End-Functionalized Microbial Poly(hydroxyalkanoate) s. ACS Sustain. Chem. Eng. 2019, 7, 7969–7978. [Google Scholar] [CrossRef]
- Xu, P.; Cao, Y.; Lv, P.; Ma, P.; Dong, W.; Bai, H.; Wang, W.; Du, M.; Chen, M. Enhanced crystallization kinetics of bacterially synthesized poly(3-hydroxybutyrate-co-3-hydroxyhexanate) with structural optimization of oxalamide compounds as nucleators. Polym. Degrad. Stab. 2018, 154, 170–176. [Google Scholar] [CrossRef]
- Gamba, A.; Fonseca, J.S.; Mendez, D.; Viloria, A.; Fajardo, D.; Moreno, N.; Rojas, I.C. Assessment of Different Plasticizer–Polyhydroxyalkanoate Mixtures to Obtain Biodegradable Polymeric Films. Chem. Eng. Trans. 2017, 57, 1363–1368. [Google Scholar]
- Fenollar, O.; Sanchez-Nacher, L.; Garcia-Sanoguera, D.; López, J.; Balart, R. The effect of the curing time and temperature on final properties of flexible PVC with an epoxidized fatty acid ester as natural-based plasticizer. J. Mater. Sci. 2009, 44, 3702–3711. [Google Scholar] [CrossRef]
- Gassner, F.; Owen, A. Physical properties of poly(β-hydroxybutyrate)-poly(ε-caprolactone) blends. Polymer 1994, 35, 2233–2236. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Ferri, J.; Boronat, T.; López-Martínez, J.; Balart, R. Processing and characterization of binary poly(hydroxybutyrate)(PHB) and poly(caprolactone)(PCL) blends with improved impact properties. Polym. Bull. 2016, 73, 3333–3350. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Garcia, D.; Garcia-Sanoguera, D.; Fombuena, V.; Lopez-Martinez, J.; Balart, R. Improvement of mechanical and thermal properties of poly(3-hydroxybutyrate)(PHB) blends with surface-modified halloysite nanotubes (HNT). Appl. Clay Sci. 2018, 162, 487–498. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Lopez-Martinez, J.; Balart, R.; Strömberg, E.; Moriana, R. Reinforcing capability of cellulose nanocrystals obtained from pine cones in a biodegradable poly(3-hydroxybutyrate)/poly(ε-caprolactone)(PHB/PCL) thermoplastic blend. Eur. Polym. J. 2018, 104, 10–18. [Google Scholar] [CrossRef]
- Garcia-Campo, M.J.; Quiles-Carrillo, L.; Masia, J.; Reig-Pérez, M.J.; Montanes, N.; Balart, R. Environmentally friendly compatibilizers from soybean oil for ternary blends of poly(lactic acid)-PLA, poly(ε-caprolactone)-PCL and poly(3-hydroxybutyrate)-PHB. Materials 2017, 10, 1339. [Google Scholar] [CrossRef] [Green Version]
- Ferri, J.M.; Fenollar, O.; Jorda-Vilaplana, A.; García-Sanoguera, D.; Balart, R. Effect of miscibility on mechanical and thermal properties of poly(lactic acid)/polycaprolactone blends. Polym. Int. 2016, 65, 453–463. [Google Scholar] [CrossRef]
- Arifin, W.; Kuboki, T. Effects of thermoplastic elastomers on mechanical and thermal properties of glass fiber reinforced poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) composites. Polym. Compos. 2018, 39, E1331–E1345. [Google Scholar] [CrossRef]
- Simões, C.; Viana, J.; Cunha, A. Mechanical properties of poly(ε-caprolactone) and poly(lactic acid) blends. J. Appl. Polym. Sci. 2009, 112, 345–352. [Google Scholar] [CrossRef]
- Katsumata, K.; Saito, T.; Yu, F.; Nakamura, N.; Inoue, Y. The toughening effect of a small amount of poly(ɛ-caprolactone) on the mechanical properties of the poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/PCL blend. Polym. J. 2011, 43, 484–492. [Google Scholar] [CrossRef] [Green Version]
- Antunes, M.C.M.; Felisberti, M.I. Blends of poly(hydroxybutyrate) and poly(epsilon-caprolactone) obtained from melting mixture. Polímeros 2005, 15, 134–138. [Google Scholar] [CrossRef] [Green Version]
- Przybysz, M.; Marć, M.; Klein, M.; Saeb, M.R.; Formela, K. Structural, mechanical and thermal behavior assessments of PCL/PHB blends reactively compatibilized with organic peroxides. Polym. Test. 2018, 67, 513–521. [Google Scholar] [CrossRef]
- Garcia, D.; Balart, R.; Sanchez, L.; Lopez, J. Compatibility of recycled PVC/ABS blends. Effect of previous degradation. Polym. Eng. Sci. 2007, 47, 789–796. [Google Scholar] [CrossRef]
- Ding, C.; Cheng, B.; Wu, Q. DSC analysis of isothermally melt-crystallized bacterial poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) films. J. Therm. Anal. Calorim. 2011, 103, 1001–1006. [Google Scholar] [CrossRef]
- Hosoda, N.; Tsujimoto, T.; Uyama, H. Green composite of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) reinforced with porous cellulose. ACS Sustain. Chem. Eng. 2014, 2, 248–253. [Google Scholar] [CrossRef]
- Burgos, N.; Tolaguera, D.; Fiori, S.; Jiménez, A. Synthesis and characterization of lactic acid oligomers: Evaluation of performance as poly(lactic acid) plasticizers. J. Polym. Environ. 2014, 22, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Avolio, R.; Castaldo, R.; Gentile, G.; Ambrogi, V.; Fiori, S.; Avella, M.; Cocca, M.; Errico, M.E. Plasticization of poly(lactic acid) through blending with oligomers of lactic acid: Effect of the physical aging on properties. Eur. Polym. J. 2015, 66, 533–542. [Google Scholar] [CrossRef]
- Hinüber, C.; Häussler, L.; Vogel, R.; Brünig, H.; Heinrich, G.; Werner, C. Hollow fibers made from a poly(3-hydroxybutyrate)/poly-ε-caprolactone blend. Express Polym. Lett. 2011, 5, 643–652. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Duart, S.; Montanes, N.; Torres-Giner, S.; Balart, R. Enhancement of the mechanical and thermal properties of injection-molded polylactide parts by the addition of acrylated epoxidized soybean oil. Mater. Des. 2018, 140, 54–63. [Google Scholar] [CrossRef]
- Ferri, J.; Garcia-Garcia, D.; Carbonell-Verdu, A.; Fenollar, O.; Balart, R. Poly(lactic acid) formulations with improved toughness by physical blending with thermoplastic starch. J. Appl. Polym. Sci. 2018, 135, 45751. [Google Scholar] [CrossRef] [Green Version]
- Quiles-Carrillo, L.; Blanes-Martínez, M.; Montanes, N.; Fenollar, O.; Torres-Giner, S.; Balart, R. Reactive toughening of injection-molded polylactide pieces using maleinized hemp seed oil. Eur. Polym. J. 2018, 98, 402–410. [Google Scholar] [CrossRef]










| Code | PHBH (wt %) | PCL (wt %) |
|---|---|---|
| 100PHBH-0PCL | 100 | 0 |
| 90PHBH-10PCL | 90 | 10 |
| 80PHBH-20PCL | 80 | 20 |
| 70PHBH-30PCL | 70 | 30 |
| 60PHBH-40PCL | 60 | 40 |
| 0PHBH-100PCL | 0 | 100 |
| Code | Tm_PCL (°C) | ΔHm_PCL (J·g−1) | ΔHm_PCL * (J·g−1) | χc_PCL (%) | Tm_PHBH (°C) | ΔHm_PHBH (J·g−1) | ΔHm_PHBH * (J·g−1) | χc_PHBH (%) |
|---|---|---|---|---|---|---|---|---|
| 100PHBH-0PCL | - | - | - | - | 138.3 | 18.92 | 18.92 | 13.0 |
| 90PHBH-10PCL | 58.0 | 6.05 | 60.52 | 38.6 | 134.0 | 19.72 | 21.91 | 15.0 |
| 80PHBH-20PCL | 59.5 | 13.41 | 67.00 | 42.7 | 134.0 | 19.70 | 24.62 | 16.8 |
| 70PHBH-30PCL | 60.8 | 17.34 | 57.70 | 36.8 | 135.0 | 18.69 | 26.68 | 18.2 |
| 60PHBH-40PCL | 60.1 | 25.27 | 63.21 | 40.3 | 135.0 | 15.91 | 26.51 | 18.1 |
| 0PHBH-100PCL | 62.0 | 72.23 | 72.23 | 46.0 | - | - | - | - |
| Code | Tg_PHBH (°C) | Tm_PCL (°C) | ΔHm_PCL (°C) | ΔHm_PCL * (°C) | Tm1_PHBH (°C) | Tm2_PHBH (°C) | ΔHm_PHBH (°C) | ΔHm_PHBH * (°C) |
|---|---|---|---|---|---|---|---|---|
| 100PHBH-0PCL | 0.46 | - | - | - | 112.8 | 137.8 | 24.9 | 24.9 |
| 90PHBH-10PCL | 0.12 | 54.6 | ** | ** | 114.5 | 138.0 | 28.4 | 31.5 |
| 80PHBH-20PCL | −0.17 | 55.0 | ** | ** | 113.5 | 139.0 | 26.2 | 32.7 |
| 70PHBH-30PCL | 0.59 | 56.0 | ** | ** | 112.4 | 141.3 | 20.3 | 28.9 |
| 60PHBH-40PCL | −0.46 | 55.6 | ** | ** | 114.8 | 139.4 | 15.4 | 25.7 |
| 0PHBH-100PCL | - | 57.0 | 45.7 | 45.7 | - | - | - | - |
| Code | CLTE below Tg_PHBH (μm·m−1·°C−1) | CLTE above Tg_PHBH (μm·m−1·°C−1) |
|---|---|---|
| 100PHBH-0PCL | 68.0 ± 1.2 | 172.5 ± 2.8 |
| 90PHBH-10PCL | 70.7 ± 1.5 | 175.6 ± 2.7 |
| 80PHBH-20PCL | 93.4 ± 1.1 | 178.6 ± 2.2 |
| 70PHBH-30PCL | 104.6 ± 1.0 | 196.6 ± 3.5 |
| 60PHBH-40PCL | 106.9 ± 0.5 | 198.9 ± 3.0 |
| Code | σt (MPa) | Et (MPa) | εb(%) | σf (MPa) | Ef (MPa) | Shore D Hardness |
|---|---|---|---|---|---|---|
| 100PHBH-0PCL | 16.0 ± 0.9 | 1022 ± 412 | 13.9 ± 1.3 | 29.5 ± 0.6 | 1029 ± 31 | 61.0 ± 0.8 |
| 90PHBH-10PCL | 14.4 ± 0.9 | 966 ± 22 | 19.4 ± 0.8 | 30.2 ± 1.7 | 966 ± 36 | 59.0 ± 0.8 |
| 80PHBH-20PCL | 13.4 ± 0.7 | 837 ± 29 | 67.9 ± 4.1 | 29.3 ± 1.5 | 946 ± 47 | 58.4 ± 1.1 |
| 70PHBH-30PCL | 13.3 ± 1.2 | 817 ± 29 | 308.3 ± 3.6 | 29.2 ± 1.0 | 813 ± 20 | 58.3 ± 0.6 |
| 60PHBH-40PCL | 14.0 ± 0.5 | 722 ± 52 | 461.0 ± 4.1 | 28.3 ± 1.1 | 802 ± 64 | 58.0 ± 0.1 |
| 0PHBH-100PCL | 12.2 ± 0.9 | 386 ± 22 | No break | 22.3 ± 0.3 | 354 ± 26 | 55.0 ± 2.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivorra-Martinez, J.; Verdu, I.; Fenollar, O.; Sanchez-Nacher, L.; Balart, R.; Quiles-Carrillo, L. Manufacturing and Properties of Binary Blend from Bacterial Polyester Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) and Poly(caprolactone) with Improved Toughness. Polymers 2020, 12, 1118. https://doi.org/10.3390/polym12051118
Ivorra-Martinez J, Verdu I, Fenollar O, Sanchez-Nacher L, Balart R, Quiles-Carrillo L. Manufacturing and Properties of Binary Blend from Bacterial Polyester Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) and Poly(caprolactone) with Improved Toughness. Polymers. 2020; 12(5):1118. https://doi.org/10.3390/polym12051118
Chicago/Turabian StyleIvorra-Martinez, Juan, Isabel Verdu, Octavio Fenollar, Lourdes Sanchez-Nacher, Rafael Balart, and Luis Quiles-Carrillo. 2020. "Manufacturing and Properties of Binary Blend from Bacterial Polyester Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) and Poly(caprolactone) with Improved Toughness" Polymers 12, no. 5: 1118. https://doi.org/10.3390/polym12051118
APA StyleIvorra-Martinez, J., Verdu, I., Fenollar, O., Sanchez-Nacher, L., Balart, R., & Quiles-Carrillo, L. (2020). Manufacturing and Properties of Binary Blend from Bacterial Polyester Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) and Poly(caprolactone) with Improved Toughness. Polymers, 12(5), 1118. https://doi.org/10.3390/polym12051118