Maleamic Acid as an Organic Anode Material in Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Material Characterization
2.3. Assessment of Electrochemical Properties
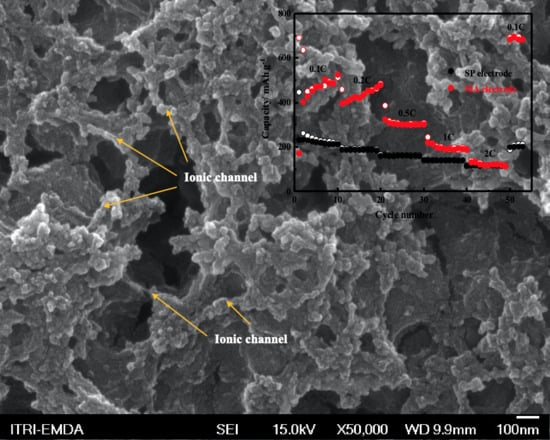
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [Green Version]
- Goodenough, J.B.; Park, K.-S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Moriwake, H.; Kuwabara, A.; Fisher, C.A.; Ikuhara, Y. Why is sodium-intercalated graphite unstable? RSC Adv. 2017, 7, 36550–36554. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: A review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Kahsay, B.A.; Ramar, A.; Wang, F.-M.; Yeh, N.-H.; Lin, P.-L.; Luo, Z.-J.; Chan, T.-S.; Su, C.-H. Investigating an all-organic battery using polyisothianaphthene as a redox-active bipolar electrode material. J. Power Sources 2019, 428, 115–123. [Google Scholar] [CrossRef]
- Muench, S.; Wild, A.; Friebe, C.; Häupler, B.; Janoschka, T.; Schubert, U.S. Polymer-Based Organic Batteries. Chem. Rev. 2016, 116, 9438–9484. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Jia, Z.; Lin, N.; Langer, T.; Lux, S.; Lund, I.; Gentschev, A.-C.; Qiao, J.; Liu, G. Molecular spring enabled high-performance anode for lithium ion batteries. Polymers 2017, 9, 657. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Cui, Y.; Bhargav, A.; Losovyj, Y.; Siegel, A.; Agarwal, M.; Ma, Y.; Fu, Y. Organotrisulfide: A high capacity cathode material for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2016, 55, 10027–10031. [Google Scholar] [CrossRef]
- Nakahara, K.; Iwasa, S.; Satoh, M.; Morioka, Y.; Iriyama, J.; Suguro, M.; Hasegawa, E. Rechargeable batteries with organic radical cathodes. Chem. Phys. Lett. 2002, 359, 351–354. [Google Scholar] [CrossRef]
- Song, Z.; Zhou, H. Towards sustainable and versatile energy storage devices: An overview of organic electrode materials. Energy Environ. Sci. 2013, 6, 2280–2301. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Sun, Y.Y.; Du, S.X.; Gao, H.J.; Zhang, S.B. Organic salts as super-high rate capability materials for lithium-ion batteries. Appl. Phys. Lett. 2012, 100, 091905. [Google Scholar] [CrossRef] [Green Version]
- Luo, C.; Borodin, O.; Ji, X.; Hou, S.; Gaskell, K.J.; Fan, X.; Chen, J.; Deng, T.; Wang, R.; Jiang, J.; et al. Azo compounds as a family of organic electrode materials for alkali-ion batteries. Proc. Natl. Acad. Sci. USA 2018, 115, 2004. [Google Scholar] [CrossRef] [Green Version]
- Amin, K.; Mao, L.; Wei, Z. Recent Progress in Polymeric Carbonyl-Based Electrode Materials for Lithium and Sodium Ion Batteries. Macromol. Rapid Commun. 2019, 40, 1800565. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Jafta, C.J.; Popovs, I.; Meyer, H.M.; Hachtel, J.A.; Huang, J.; Sumpter, B.G.; Dai, S.; Sun, X.-G. A dicyanobenzoquinone based cathode material for rechargeable lithium and sodium ion batteries. J. Mater. Chem. A 2019, 7, 17888–17895. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Q.; Li, L.; Niu, Z.; Chen, J. Design strategies toward enhancing the performance of organic electrode materials in metal-ion batteries. Chem 2018, 4, 2786–2813. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Lu, Y.; Chen, J. Advanced Organic Electrode Materials for Rechargeable Sodium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1601792. [Google Scholar] [CrossRef]
- Häupler, B.; Wild, A.; Schubert, U.S. Carbonyls: Powerful Organic Materials for Secondary Batteries. Adv. Energy Mater. 2015, 5, 1402034. [Google Scholar] [CrossRef]
- Sharma, P.; Damien, D.; Nagarajan, K.; Shaijumon, M.M.; Hariharan, M. Perylene-polyimide-based organic electrode materials for rechargeable lithium batteries. J. Phys. Chem. Lett. 2013, 4, 3192–3197. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, K.; Zhao, Q.; Pei, L.; Chen, J. High-performance sodium batteries with the 9,10-anthraquinone/CMK-3 cathode and an ether-based electrolyte. ChemComm 2015, 51, 10244–10247. [Google Scholar] [CrossRef]
- Huang, W.; Zhu, Z.; Wang, L.; Wang, S.; Li, H.; Tao, Z.; Shi, J.; Guan, L.; Chen, J. Quasi-Solid-State Rechargeable Lithium-Ion Batteries with a Calix [4] quinone Cathode and Gel Polymer Electrolyte. Angew. Chem. Int. Ed. 2013, 52, 9162–9166. [Google Scholar] [CrossRef]
- Armand, M.; Grugeon, S.; Vezin, H.; Laruelle, S.; Ribière, P.; Poizot, P.; Tarascon, J.M. Conjugated dicarboxylate anodes for Li-ion batteries. Nat. Mater. 2009, 8, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Liu, J.; Mahurin, S.; Dai, S.; Guo, Z.; Sun, X.-G. Polythiophene coated aromatic polyimide enabled ultrafast and sustainable lithium ion batteries. J. Mater. Chem. A 2017, 5, 24083–24090. [Google Scholar] [CrossRef]
- Siwal, S.S.; Zhang, Q.; Devi, N.; Thakur, V.K. Carbon-based polymer nanocomposite for high-performance energy storage applications. Polymers 2020, 12, 505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, H.; Li, P.; Liu, J.; Mahurin, S.; Chen, J.; Hensley, D.K.; Veith, G.M.; Guo, Z.; Dai, S.; Sun, X.G. Aromatic polyimide/graphene composite organic cathodes for fast and sustainable lithium-ion batteries. ChemSusChem 2018, 11, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, I.A.; Yuan, Y.; Bommier, C.; Wang, X.; Ma, L.; Leonard, D.P.; Lerner, M.M.; Carter, R.G.; Wu, T.; Greaney, P.A.; et al. Mg-Ion Battery Electrode: An Organic Solid’s Herringbone Structure Squeezed upon Mg-Ion Insertion. J. Am. Chem. Soc. 2017, 139, 13031–13037. [Google Scholar] [CrossRef]
- Fédèle, L.; Sauvage, F.; Bois, J.; Tarascon, J.-M.; Bécuwe, M. Lithium insertion/de-insertion properties of π-extended naphthyl-based dicarboxylate electrode synthesized by freeze-drying. J. Electrochem. 2014, 161, A46–A52. [Google Scholar] [CrossRef]
- Chen, H.; Armand, M.; Demailly, G.; Dolhem, F.; Poizot, P.; Tarascon, J.M. From biomass to a renewable LixC6O6 organic electrode for sustainable Li-ion batteries. ChemSusChem 2008, 1, 348–355. [Google Scholar] [CrossRef]
- Walker, W.; Grugeon, S.; Vezin, H.; Laruelle, S.; Armand, M.; Tarascon, J.M.; Wudl, F. The effect of length and cis/trans relationship of conjugated pathway on secondary battery performance in organolithium electrodes. Electrochem. Commun. 2010, 12, 1348–1351. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, C.; Lu, Y.; Liu, L.; Liang, J.; Chen, J. Rechargeable Lithium Batteries with Electrodes of Small Organic Carbonyl Salts and Advanced Electrolytes. Ind. Eng. Chem. 2016, 55, 5795–5804. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, Y.; Qu, Q.; Zheng, X.; Zhang, J.; Liu, G.; Battaglia, V.S.; Zheng, H. Ultrahigh-capacity organic anode with high-rate capability and long cycle life for lithium-ion batteries. ACS Energy Lett. 2017, 2, 2140–2148. [Google Scholar] [CrossRef]
- Song, Z.; Qian, Y.; Liu, X.; Zhang, T.; Zhu, Y.; Yu, H.; Otani, M.; Zhou, H. A quinone-based oligomeric lithium salt for superior Li–organic batteries. Energy Environ. Sci. 2014, 7, 4077–4086. [Google Scholar] [CrossRef]
- Cui, H.; Li, Q.; Qiu, G.; Wang, J. Carbon-chain inserting effect on electronic behavior of single-walled carbon nanotubes: A density functional theory study. MRS Commun. 2018, 8, 189–193. [Google Scholar] [CrossRef]
- Peng, B.; Xu, Y.; Wang, X.; Shi, X.; Mulder, F.M. The electrochemical performance of super P carbon black in reversible Li/Na ion uptake. Sci. China Phys. Mech. 2017, 60, 064611. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L., III. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef] [Green Version]
- Goriparti, S.; Harish, M.N.K.; Sampath, S. Ellagic acid—A novel organic electrode material for high capacity lithium ion batteries. ChemComm 2013, 49, 7234–7236. [Google Scholar] [CrossRef] [PubMed]
- Abouimrane, A.; Weng, W.; Eltayeb, H.; Cui, Y.; Niklas, J.; Poluektov, O.; Amine, K. Sodium insertion in carboxylate based materials and their application in 3.6 V full sodium cells. Energy Environ. Sci. 2012, 5, 9632–9638. [Google Scholar] [CrossRef]
- Deng, Q.; Xue, J.; Zou, W.; Wang, L.; Zhou, A.; Li, J. The electrochemical behaviors of Li2C8H4O6 and its corresponding organic acid C8H6O6 as anodes for Li-ion batteries. J. Electroanal. Chem. 2016, 761, 74–79. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Zhang, K.; Zhu, Z.; Tao, Z.; Chen, J. Organic Li4C8H2O6 nanosheets for lithium-ion batteries. Nano Lett. 2013, 13, 4404–4409. [Google Scholar] [CrossRef]
- Zhao, R.R.; Cao, Y.L.; Ai, X.P.; Yang, H.X. Reversible Li and Na storage behaviors of perylenetetracarboxylates as organic anodes for Li- and Na-ion batteries. J. Electroanal. Chem. 2013, 688, 93–97. [Google Scholar] [CrossRef]
- Chen, L.; Liu, S.; Zhao, L.; Zhao, Y. OH-substituted 2, 3-dichloro-5, 6-dicyano-1, 4-benzoquinone as highly stable organic electrode for lithium ion battery. Electrochim. Acta 2017, 258, 677–683. [Google Scholar] [CrossRef]
- Światowska, J.; Lair, V.; Pereira-Nabais, C.; Cote, G.; Marcus, P.; Chagnes, A. XPS, XRD and SEM characterization of a thin ceria layer deposited onto graphite electrode for application in lithium-ion batteries. Appl. Surf. 2011, 257, 9110–9119. [Google Scholar] [CrossRef]
- Zhao, L.; Watanabe, I.; Doi, T.; Okada, S.; Yamaki, J.-I. TG-MS analysis of solid electrolyte interphase (SEI) on graphite negative-electrode in lithium-ion batteries. J. Power Sources. 2006, 161, 1275–1280. [Google Scholar] [CrossRef]
- Dedryvère, R.; Leroy, S.; Martinez, H.; Blanchard, F.; Lemordant, D.; Gonbeau, D. XPS valence characterization of lithium salts as a tool to study electrode/electrolyte interfaces of Li-ion batteries. J. Phys. Chem. B 2006, 110, 12986–12992. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atsbeha Kahsay, B.; Wang, F.-M.; Hailu, A.G.; Su, C.-H. Maleamic Acid as an Organic Anode Material in Lithium-Ion Batteries. Polymers 2020, 12, 1109. https://doi.org/10.3390/polym12051109
Atsbeha Kahsay B, Wang F-M, Hailu AG, Su C-H. Maleamic Acid as an Organic Anode Material in Lithium-Ion Batteries. Polymers. 2020; 12(5):1109. https://doi.org/10.3390/polym12051109
Chicago/Turabian StyleAtsbeha Kahsay, Berhanemeskel, Fu-Ming Wang, Alem Gebrelibanos Hailu, and Chia-Hung Su. 2020. "Maleamic Acid as an Organic Anode Material in Lithium-Ion Batteries" Polymers 12, no. 5: 1109. https://doi.org/10.3390/polym12051109
APA StyleAtsbeha Kahsay, B., Wang, F. -M., Hailu, A. G., & Su, C. -H. (2020). Maleamic Acid as an Organic Anode Material in Lithium-Ion Batteries. Polymers, 12(5), 1109. https://doi.org/10.3390/polym12051109