3.1. Evolution of Surface Wettability
Polymer treatments were performed at a constant distance between the exit of the dielectric tube and the polymer surface of 5 mm and for various treatment times. Just after the treatment, the samples were probed for wettability using our multi-droplet device. The results are shown graphically in
Figure 2.
The left column in
Figure 2 is a 2D/3D plot of water contact angles for the case when PS samples were covered with the MgF
2 optical window and thus exposed to UV-VUV radiation only. The right column in
Figure 2 represents results without the MgF
2 window when the samples were exposed directly to the plasma jet. Treatment times in all rows in
Figure 2 were the same for covered and uncovered samples. At a glance, one can observe a saturation of the surface wettability for the case of direct plasma treatment, and a graduate increase of the surface wettability in the case of treatment by VUV radiation only. The water contact angle of the untreated PS was approximately 90°. The treatment by UV-VUV radiation for 30 s (
Figure 2a) revealed a rather small spot of activated PS right in the center. As shown in
Figure 1, the sample was positioned in such a way that it was centered on the axis of the discharge tube. The yellow spot on the surface treated for 30 s appeared at the position of the plasma jet. As mentioned earlier, the inner diameter of the dielectric tube is approximately 3 mm, and so is the diameter of the activated spot on the PS treated by the radiation only for 30 s. This observation indicates that the radiation suitable for surface activation is focused in the same way as plasma itself. The radiation, of course, occurs over the entire length of the plasma jet, and the excited atoms or molecules radiate in all directions. The fact that the activated spot is rather well-focused indicates that the radiation that should pass the effluent region away from the axis of the gas jet does not contribute to surface activation. This is a consequence of a rather short absorption length, which is of the order of 100 μm [
11].
A double treatment time (60 s) shows improved wettability, as indicated in
Figure 2b. The diameter of the activated spot remains practically the same, but the water contact angles at the center of the sample are much smaller. The interface between the region of no activation and the area with low water contact angles is very sharp. The reason is probably the poor transparency of the ambient atmosphere for radiation arising from gaseous plasma.
The prolonged treatment by radiation, i.e., after 180 s, causes a further increase of the surface wettability in the center of the activated spot, as revealed in
Figure 2c. Simultaneously, the spot size increases, indicating that the VUV radiation is not perfectly focused. Such a long treatment time is, therefore, sufficient for increasing the size of the activated spot over the diameter of the dielectric tube. Weakly activated areas are also observed outside the spot. The appearance of such rather unexpected observation is explained by considering the result of PS treated for 600 s, which is shown in
Figure 2d. As already mentioned, the MgF
2 window was smaller than the size of the sample; therefore, only the central part was covered. In
Figure 2d, we observe a central spot of a well-activated PS and a halo stretching far away from the axis. In-between, there is a ring-like area of poor wettability. The diameter of the ring coincides with the diameter of the MgF
2 window. The spot of the well-activated surface, which was fully covered with the MgF
2 window, is obviously because of a large fluence of VUV radiation. However, the halo formed on the uncovered PS area is probably because of the interaction of reactive chemical species present away from the axis of the experimental setup that may rich the uncovered PS surface. The halo is therefore explained by weak activation of air molecules away from the main Ar jet.
The right-hand column in
Figure 2e–h shows a less pronounced evolution of the surface wettability with treatment time when no optical covering was used. Still, the area of the low water contact angle after 600 s of treatment (
Figure 2h) is approximately double as compared to 30 s (
Figure 2e). The results show that the minimum achievable contact angle of approximately 13° is obtained on the spot much larger than the jet diameter already after 30 s of plasma treatment. The prolonged treatment causes enlargement of the spot size, but the interface between non-treated and well-activated surface remains fairly unchanged. Such observation is typical for the evolution of any surface property where saturation occurs.
3.2. Evolution of Chemical Modifications
The samples whose wettability was elaborated in
Figure 2 were also characterized by XPS. Because of the time-consuming XPS measurements, the XPS spectra were acquired only in the center of the polymer sample, which was aligned with the axis of the APPJ jet. The XPS surface composition versus treatment time is presented in
Table 1 and
Table 2.
Table 1 represents the surface composition versus treatment time for the case of samples covered with the MgF
2 window. Even the untreated PS contains about 2 at. % of oxygen, which is probably because of the weak oxidation of the polymer foil before plasma treatment. The treatment for 30 s shows enrichment of PS surface with oxygen as well as some nitrogen. The concentration of oxygen increases with increasing treatment time, as indicated in
Table 1. The increase is rather monotonous. The final concentration of 30 at. % of oxygen is comparable to the best results observed by low-pressure oxygen plasma treatment [
2,
15]. The increase of oxygen concentration versus treatment time is in agreement with the surface wettability shown in
Figure 2a–d, where we can also observe a gradual increase with treatment time.
Interesting is the evolution of the O/C ratio of samples treated with radiation only versus the treatment time, which is shown in
Figure 3. One can observe a highly monotonous approaching of saturation, which occurs at the O/C ratio close to 0.45. In the same figure, there is also the curve for the minimum WCA measured in the center of the PS samples. The trends of WCA and surface composition are opposite as expected and reported already by other authors [
16,
17,
18]. Additionally, in
Figure 4 is shown a correlation between the minimum WCA versus the XPS O/C ratio. One can observe a monotonous decrease. In
Figure 4, we used a linear fitting of the measured points for eye guidance only.
Table 2 represents the concentration of different elements in the center of the samples treated directly by the plasma jet. Opposite to results presented in
Table 1, both oxygen and nitrogen concentrations in
Table 2 remained practically unchanged with treatment time. This observation is sound with the right column of
Figure 2e–h, which shows the saturation of surface wettability in sample’ centers already at the shortest treatment time. What is more interesting is the fact that the observed O/C ratio in
Table 2 is significantly lower than in
Table 1. The graphical presentation of the results of surface functionalization and wettability versus the treatment time is shown in
Figure 5. One can observe almost perfect saturation of both surface properties. The rather poor oxidation of the PS sample upon direct exposure to APPJ as compared to treatment by VUV radiation is worth discussing. Such an observation was already reported by Oehrline [
7] where better functionalization was observed for samples treated by remote plasma jet, i.e., outside the glowing plasma. The authors [
7] concluded that long-lived species, such as singlet oxygen molecules, may be more important for surface activation than short-lived species such as O atoms. Furthermore, they proposed an important effect of high-energy photons. The same group also reported etching upon treatment of PS with plasma rich in reactive species [
6]. The latter effect can explain a rather poor surface functionalization when using direct plasma treatment, as observed in our case. As is already known for low-pressure plasma, the plasma species cause both chemical and physical interaction with the polymer surface [
15]. Excessive energy stimulates more extensive chemical reactions, which probably results in the formation of oxygen-rich molecular fragments, which may desorb from the surface. This effect can be avoided efficiently in the case of low-pressure treatment by using late afterglows instead of gaseous plasma. In the case of a direct treatment by plasma jet, the mechanisms of interaction between O atoms and polymer surfaces are difficult to evaluate because of the huge gradients of reactive species.
The treatment by VUV radiation from the APPJ is more efficient than by direct plasma treatment as long as the concentration of oxygen, as determined by XPS, is the merit. The VUV is known to break bonds in organic materials, and dangling bonds are attacked by gaseous molecules [
19]. Dangling bonds are very reactive, so even exposure to molecular oxygen or nitrogen causes functionalization. Interestingly enough, the water contact angle remains rather large even after prolonged treatment. The WCA for samples treated directly by plasma jet stabilizes at about 20° (
Figure 5), whereas for VUV treatment it keeps decreasing slowly, but remains over 25° even after 10 min (
Figure 3). Here it is worth mentioning that the surface wettability reflects both the surface functionalization and roughness, whereas XPS reveals only somehow averaged concentration of an element over the probing depth. While plasma species do not penetrate deep into the polymer, the VUV radiation has a penetration depth typically much larger than the probing depth of XPS [
20]. One possible explanation of the paradox could be the modification of a thicker surface film by the VUV. Although VUV has been proved to cause also etching [
19], the etching rate is much lower than for the case of etching with plasma radicals. Such a rather poor etching enables the preservation of highly saturated functional groups on the polymer surface and thus the ability for better functionalization of the subsurface layer. Another possible explanation of the paradox is different surface roughness. It was already shown that etching causes higher surface roughness [
6], and an increase in roughness has an effect on the surface wettability [
21].
Different evolutions of XPS oxygen concentration shown in
Table 1 and
Table 2 are reflected in a different degree of surface functionalization of samples treated by VUV or by direct plasma treatment. The evolution of surface functional groups is revealed from the high-resolution C1s spectra of samples shown in
Figure 6 and
Figure 7.
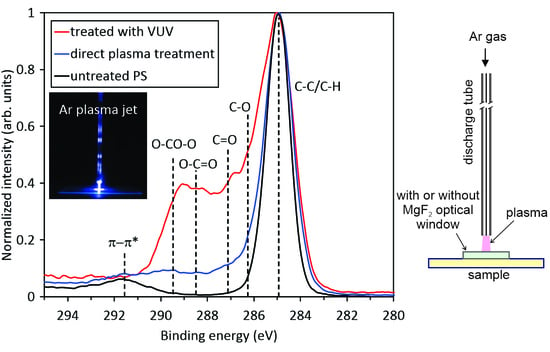
Figure 6 represents the high-resolution XPS spectra of samples treated by direct exposure to the plasma jet. The untreated sample shows only one peak at about 285 eV attributed to C–C/C–H. Another peak at 291.5 eV corresponds to the shake-up peak reflecting the aromatic character of this polymer. The high-resolution spectra were acquired for all treatment times. One can observe almost perfect overlapping of the curves. The result is expected, taking into account the saturation of the polymer surface as revealed from
Table 2 and
Figure 5, as well as
Figure 2e–h. Interestingly enough, no specific functional group predominates on the surface of the samples treated directly by APPJ.
More interesting is the evolution of the surface functionalities for samples treated by VUV radiation only, which is shown in
Figure 7. Treatment by 30 s definitely causes some changes, but they are rather similar to those observed in
Figure 6. However, 1 min of treatment already reveals a substantial formation of highly-oxidized functional groups. Finally, after 10 min of VUV radiation, a well-distinguished peak occurs at the binding energy of ~289 eV, which corresponds to carboxyl and carbonate groups, as explained below. This significant difference between
Figure 6 and
Figure 7 regarding the evolution of functional groups on the PS sample treated by VUV radiation or treated directly by APPJ is further supported by
Figure 8 and
Table 3. In
Figure 8 is shown a comparison of a fitting of C1s peaks for the longest treatment time (10 min) for both treatment conditions, where the differences are the most noticeable. As already mentioned above, high concentrations of highly oxidized carbon functional groups are formed for the case of VUV treatment. More detailed evolution of specific functional groups for the samples treated by VUV radiation is shown in
Table 3. For comparison, also direct plasma treatment is shown, but only for 10 min of treatment because the surface is already saturated at short treatment times (
Figure 6). In
Table 3, we can observe significant destruction of the aromatic ring in the case of VUV treatment, which is related to the formation of highly oxidized carbon groups. Such aromatic ring opening and formation of carbonate groups for PS have already been reported in the literature for low-pressure plasma treatments [
2,
15] and for the remote (afterglow) APPJ treatment [
7], even though it is easier to oxidize and break the aliphatic chain than the aromatic one.
The effect of PS treatment by VUV radiation is thus more similar to the effect of low-pressure gaseous plasma (or afterglow) treatment than to APPJ [
1,
2,
15]. The possible explanation of this observation is that the power density at atmospheric pressure plasma is by far larger than in low-pressure plasma useful for surface activation of polymers. According to [
1,
2,
15], the best results in terms of surface activation of polymers by low-pressure plasma treatment are observed using weakly ionized gaseous plasma such as electrodeless RF discharge in the capacitive mode. In such plasma, the kinetic effects are entirely negligible as compared to chemical effects (as long as the samples are kept at a floating potential), because the dissociation fraction is orders of magnitude larger than ionization. Highly ionized low-pressure plasmas do not allow for optimal surface functionalization due to extensive etching. Taking into account these considerations, the rather poor functionalization of PS samples upon direct exposure to glowing APPJ (
Figure 6) is a consequence of the simultaneous etching of highly polar oxygen compounds.