1. Introduction
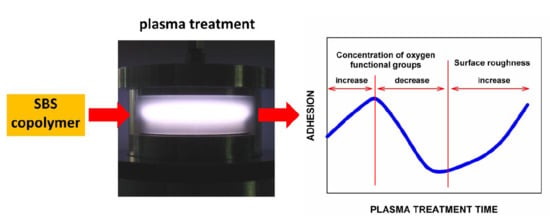
In general, the adhesive bonding of polymers to themselves as well as to other materials presents an important problem, especially in the case of elastomers and rubbers used in the industry. The strength and quality of such adhesive-bonded joints depend, to a great extent, on the chemical structure and morphology of the polymer surface, and therefore can be controlled by various surface treatments. It is already known for more than 20 years that a particularly useful method in this respect is the low-temperature (non-equilibrium), both low pressure and atmospheric pressure, plasma treatment [
1,
2,
3,
4,
5,
6,
7]. Such treatment, which can be performed either in inert plasmas (generated e.g., in Ar or He) or chemically reactive (but non-polymerizable) plasmas (generated e.g., in O
2, CO
2, or H
2O), causes changes in the chemical structure of the polymer surface by two fundamental processes. The first one consists in the cleavage of chemical bonds on the surface, which leads to the creation of radical states. On the states, functional groups, such as hydroxyl (−OH), carbonyl (>C=O), etc., can be formed directly in the plasma processes (from atoms present in the reactive plasmas) or further after the contact of the surface with air atmosphere (in the case of inert plasmas). The radical centers are also responsible for the formation of cross-linking bonds (for example, C−C, C−O−C), the number of which increases with the concentration of these centers. In turn, the second process involves bombarding the surface with ions, which leads to the preferential etching of the surface by removing certain atoms or their groups and, consequently, increases the surface roughness [
4].
Usually, after the plasma treatment, a pronounced increase in the adhesion of the polymer surfaces to adhesives was observed. In some cases, only a few seconds of plasma exposure was enough to obtain several times higher peel strength than that of the non-treated samples [
4]. There is no doubt that the observed improvement in adhesive properties results from changes in the chemical structure and nanomorphology of the polymer surface. It was found that the functional groups produced on the surface, on the one hand, can react chemically with adhesives, causing the chemical adhesion [
8], and on the other hand, they can enhance the surface energy (mainly the polar component of the surface energy) being responsible for the thermodynamic adhesion [
9]. The surface roughness generated during plasma treatment leads in turn to the enhancement of the mechanical adhesion [
10,
11]. Thus, it would be expected that the adhesion force should steadily increase with increasing plasma treatment time. However, it has already been reported several times that after some time of plasma treatment, the peel strength of the adhesive-bonded joints formed with the polymers begins to decrease during further processing [
9,
12,
13,
14,
15]. Besides, it was also observed that for longer treatment time, after the reduction of the peel strength, its re-increase occurred [
13].
The aim of this work is an attempt to explain, based on the kinetic approach, changes occurring on the polymer surface during the plasma treatment. As a measure of these changes on the surface, the peel strength of the adhesive-bonded joints was established. It was assumed that the value of this parameter is directly proportional to the concentration of active states responsible for the adhesion, such as appropriate functional groups and roughness. The studies were carried out on a model elastomer such as a styrene–butadiene–styrene (SBS) copolymer. Rubbers based on SBS copolymers are one of the main materials used in the footwear industry for the production of soles. Therefore, there is a wide interest in gluing these materials (mainly with the use of polyurethane adhesives) [
16,
17]. Plasma surface activation of elements made of SBS rubbers, which is a clean, waste-free, and environmentally friendly method, is currently the leading solution in this area. However, it requires further research to better understand the mechanisms of plasma activation of the surface, which has a direct impact on the practical application of this method.
3. Results and Discussion
For a more profound understanding of the polymer surface modification created by low-temperature plasma treatments, we analyzed the dependences of the peel strength for adhesive-bonded joints prepared between the SBS surface and PU adhesive on the plasma treatment time and discharge power using various types of plasma.
Figure 1a presents an example of such dependences for the CCl
4 plasma. Similar dependences for other types of plasma, but only for selected discharge powers (extreme values), are shown in
Figure 1b. As can be seen, all plasma treatments, irrespective of the type of plasma and the discharge power, produce a considerable improvement in the adhesion properties of the SBS copolymer. The peel strength increases rapidly and attains a maximum value in a relatively short time of plasma treatment. (Although this is not seen in the Ar plasma (
Figure 1b), there is no doubt that also in this case the maximum exists somewhere in time ≤ 5 s, because for time = 0 (without plasma treatment) the peel strength is only ~2.0 kN/m.) Then, a decrease in the peel strength value is visible, after which in most cases, especially for high power discharges, the peel strength increases again. Although all these relationships have the same character, their exact shape depends on the type of plasma and the discharge power. For example, the maximum value of the peel strength occurring in the initial plasma treatment period clearly shifts towards shorter times with an increase in the discharge power, but in different ways for each type of plasma used.
To explain the nature of the observed phenomena, it should first of all be emphasized that as the peel strength depends on the parameters of the plasma treatment, it proves that the SBS–PU interface is responsible for the peel test results. If SBS, PU, and leather cohesion forces or the PU–leather interface were responsible for the peel strength, peel strength should be independent of the plasma treatment and should be practically constant.
For the description of the peel strength changes as a function of the plasma treatment time, three main processes were considered: (1) the generation of radical states followed by the creation of functional groups involved in the adhesive bonding process, (2) the surface cross-linking that decreases the concentration of these functional groups, and (3) the formation of nano-roughness. In our case, the first process concerns the generation of radical states on the surface and then creating functional groups on them, such as C−OH, C−Cl, and >C=O, capable of reacting with the PU adhesive. As already mentioned, in reactive plasmas, these groups are formed directly during the plasma treatment, whereas in inert plasmas, they are formed mainly after contacting the radical states with air. The second process involves the reduction of both the existing functional groups and the sites where they could be created. In reactive plasmas, this consists in converting in plasma the already formed functional groups into groups that are inactive in relation to the adhesive (mainly as a result of crosslinking). An example of such predicted reactions for C−OH groups is shown in
Figure 2a–c. In turn, the reduction of the sites where functional groups can be created is effected by direct recombination and cross-linking between the radical states. This effect dominates in the case of inert plasmas before the polymer surface is contacted with air and the formation of functional groups begins (
Figure 2d). Thus, regardless of the type of plasma, we can observe in the second process a systematic decrease in the concentration of active functional groups that can react with the PU adhesive. The higher the concentration of radical states and/or functional groups, the easier the cross-linking process with their participation occurs.
The course of the first and second processes justifies the assumption that they are consecutive processes, each of the first order. For constant plasma parameters, the rate of the first process depends only on the concentration of surface sites ready to form radical states (for example, ~C−H → C
•). The rate of the second process, in turn, depends only on the concentration of created radical states. The resulting balanced concentration of radical states after a given plasma treatment time is, in fact, equal to the concentration of active functional groups formed on these states (during the plasma process or after contact with air), which in turn is proportional to the peel strength (
Fp’) based on the interaction between the functional groups and the PU adhesive. The integrated form of the kinetic equation describing these two consecutive processes (by analogy to the commonly known equation for the consecutive reactions, see, e.g., in [
22]) is as follows,
where
k1 and
k2 are the rate constants for the first and second processes, respectively;
A0 is a parameter proportional to the initial concentration of surface sites, on which radical states can be created; and
t is the plasma treatment time.
An example of the theoretical curve described by Equation (1) is shown in
Figure 3 (curve 1). As one can see, there is a clear maximum resulting from the competition between the process leading to the formation of functional groups and the process leading to their reduction. Such maxima, as already shown above, are observed for the dependence of the peel strength for the adhesive-bonded joints on the plasma treatment time.
Indeed, it has already been found several times that changes in the concentration of functional groups responsible for reactions with PU adhesives (C−OH, C−Cl, >C=O), measured directly by FTIR and XPS spectroscopies as a function of plasma treatment time, demonstrate a good correlation with an analogous dependence for the peel strength of adhesive joints [
4,
8,
13,
18].
Figure 4a,b shows examples of the relationships of the −OH groups’ concentration and the peel strength as functions of treatment time with O
2 plasma and He plasma, respectively.
The relative concentrations of −OH groups are expressed by normalized surface areas of the characteristic IR bands in the range of 3650 to 3710 cm
−1, which are assigned to O−H stretching vibrations [
23]. This range of IR spectrum is shown in
Figure 5a. As can be seen, the positions of the maxima on the plasma treatment time scale for the concentration of −OH groups and the peel strength of adhesive joints are in good agreement with each other. On the other hand, it was also found that the relative concentration of C−O−C bridges predicted as one of the results of the cross-linking process (shown in
Figure 2), revealed by IR bands in the range of 990 to 1130 cm
−1 (
Figure 5b), which are attributed to C−O−C stretching vibrations [
23], increases systematically with the plasma treatment time. Thus, the presented results of the IR investigations are fully consistent with the concept of two consecutive processes described by the kinetic Equation (1): the process of creation of functional groups and the process of their reduction.
The third process contributing to the strength of the adhesive joint (
Fp’’) is the process of creating roughness on the surface as a result of preferential etching. For fixed plasma parameters, that is, for a fixed reaction rate constant, the rate of this process depends only on the concentration of the sites on the surface susceptible to the etching, and therefore the process can be described by the following first-order equation,
where
k3 is the rate constant of the third process and
B0 is a parameter proportional to the initial concentration of sites on the surface, which are involved in the formation of roughness. An example of the theoretical curve described by Equation (2) is shown in
Figure 3 (curve
2). The experimental evaluation of surface roughness as a function of the plasma treatment time confirms the nature of this relation.
Figure 6 presents an example of the roughness changes on the SBS surface subjected to plasma treatment, determined from AFM measurements. In
Figure 7, several selected AFM images illustrating such changes in the roughness are shown.
Taking into account all three processes discussed above, the dependence of the total peel strength of the adhesive-bonded join (
Fp) on the plasma treatment time can be described by the overall kinetic equation, which is a combination of Equations (1) and (2). For formalities, the peel strength for the plasma non-treated surface (
F0) is also included in the equation
The theoretical curve described by Equation (3), which is a combination of curves 1 and 2, is shown in
Figure 3 (curve 3). As one can see, its shape is very similar to the experimental relationships between the peel strength and the plasma treatment time (
Figure 1). Equation (3) was therefore used to analyze all experimental results concerning the dependence of
Fp on
t obtained for the O
2, CO
2, CCl
4, Ar, and He plasmas. It was done according to a numerical curve-fitting algorithm (TableCurve, Jandel Sci., USA), which enables to determine the parameters
k1,
k2,
k3,
A0, and
B0. In general, very good fitting between the experimental results and the theoretical curves calculated from Equation (3) was obtained. The determined parameters and the coefficient of determination
r2 are given in
Table 1.
Several important conclusions can be drawn from the data presented in
Table 1. First of all, the
k1 values are drastically higher for the Ar plasma than those for all other plasmas used. This indicates that the Ar plasma, in contrast to the other plasmas, is much more effective in generating free radicals on the SBS surface. On the other hand, the rate constant for the cross-linking process (
k2) behaves differently depending on the type of plasma. For the reactive plasmas (generated in O
2, CO
2, and CCl
4), the
k2 values are very similar to the corresponding
k1 values. As the rate constants
k1 and
k2 contain information about the mechanism of plasma action, it can be assumed that both processes—the formation of active functional groups as well as their reduction—proceed by the same plasma mechanism. However, for both inert plasmas (generated in Ar and He), the rate constant
k2 has similar values, and they are practically the same for various discharge powers. The lack of dependence between the cross-linking process and plasma parameters could have been predicted taking into account the fact that in this case the direct cross-linking between the created radical states takes place, for which the plasma is no longer needed.
Similarly, the differences between the reactive and inert plasmas are visible in the preferential etching processes. For the reactive plasmas, there is a systematic increase in reactivity (k3 increases) with increasing discharge power, with the O2 plasma being the least reactive. The concentration of sites on the surface (parameter B0), which are involved in the O2 plasma etching process, is almost one order of magnitude greater than when the CO2 and CCl4 plasma is used, which proves that another etching mechanism is active in the presence of the O2 plasma. In the case of the inert plasmas, however, the correlation between the discharge power and the etching process is not as clear and requires further investigations, although the values of B0 are comparable to the ones obtained for the CO2 and CCl4 plasma, which may indicate the similarity of etching processes in the plasma of Ar, He, CO2, and CCl4.
Finally, let us pay attention to the
A0 parameter. Its values, as shown in
Table 1, which should characterize the chemical structure of the SBS surface before plasma treatment, are indeed practically independent of the plasma types and discharge powers (
A0 = 14 ± 1 kN/m). This confirms the correctness of the proposed kinetic model. Slightly higher values of
A0 than those of
B0, except for O
2 plasma, where we anticipate another etching mechanism, confirm the assumption that the concentration of bonds that can participate in the creation of free radicals should be higher than the concentration of bonds involved in the etching process. For example, cleavage of the C−H bond generates a radical center on the surface (C
•), but it is not yet sufficient to start the polymer chain scission, which initiates the formation of nano-roughness.
To more accurately determine the mechanism of the free radical generation process, the dependence of the rate constant
k1 on the discharge power was analyzed. As a basis, the power function in the following form was taken,
where
P is the discharge power, and
a and
n are the coefficients of the equation.
Figure 8 shows graphs of this dependence for all tested plasma types. In all cases, except for the O
2 plasma, the coefficient
n is equal to approximately 0.5−0.6. For the O
2 plasma, this coefficient reaches a value of 2.0.
Generally, two mechanisms can be responsible for the radical generation on the SBS surface by low-temperature (RF) plasma. The first is connected with the electron bombardment, and the second with the ion bombardment. It has been already found that the dependence of the radical formation rate on the discharge power is approximated by a square function (
n ≈ 2) when the first mechanism dominates [
24]. On the other hand, if the second mechanism determines the radical formation, the rate of the process is proportional to the average energy of ions bombarding the surface, which is, in turn, approximated by a square root function of the discharge power (
n ≈ 0.5) [
25,
26]. On this basis, it can be concluded that the ion bombardment mechanism dominates during the SBS surface treatment by inert gas plasmas (Ar and He) as well as CO
2 and CCl
4 plasmas. In the case of O
2 plasma, its interaction with the SBS surface should be associated with the electron bombardment mechanism. The different behavior of the O
2 plasma compared to other plasma types tested in this work has already been revealed in the SBS surface etching discussed above.



 ) and −OH concentration (
) and −OH concentration (  ) for O2 (20 W) plasma; (b) correlation between peel strength (
) for O2 (20 W) plasma; (b) correlation between peel strength (  ) and −OH concentration (
) and −OH concentration (  ) for He (10 W) plasma; (c) the dependence of C−O−C concentration on the time of treatment with O2 (20 W) plasma and He (10 W) plasma.
) for He (10 W) plasma; (c) the dependence of C−O−C concentration on the time of treatment with O2 (20 W) plasma and He (10 W) plasma.
 ) and −OH concentration (
) and −OH concentration (  ) for O2 (20 W) plasma; (b) correlation between peel strength (
) for O2 (20 W) plasma; (b) correlation between peel strength (  ) and −OH concentration (
) and −OH concentration (  ) for He (10 W) plasma; (c) the dependence of C−O−C concentration on the time of treatment with O2 (20 W) plasma and He (10 W) plasma.
) for He (10 W) plasma; (c) the dependence of C−O−C concentration on the time of treatment with O2 (20 W) plasma and He (10 W) plasma.