Fabrication and Characterization of Hydrogels Based on Gelatinised Collagen with Potential Application in Tissue Engineering
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Characterization of the Raw Material
2.2.1. Analysis of the Chemical Composition
2.2.2. Protein Solubility and Z-Potential
2.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.3. Formation of Hydrogels
2.4. Characterization of the Hydrogels
2.4.1. Rheological Evaluation
- Strain sweep tests: Measurements between 0.1% and 100% strain and a constant frequency of 1 Hz were performed to determine the linear viscoelastic range (interval where the elastic and viscous moduli are independent of the strain) and the critical strain (the maximum strain supported by the sample within the linear viscoelastic range). These tests were performed at 37 °C to simulate the potential behaviour of the hydrogels in the body.
- Frequency sweep tests: The measurements were carried out in a frequency range between 0.1 and 10 Hz at a specific strain for each system (within the linear viscoelastic range). In these tests, the elastic and viscous moduli (G’ and G’’, respectively) were obtained, together with the loss tangent (tan δ) and complex viscosity (η*). In a similar way than the strain sweep tests, these measurements were performed at 37 °C to simulate the potential stability of the hydrogels in the body.
- Time sweep tests: In this case, the test was carried out to evaluate the gelation process of the hydrogels. The test was performed at constant frequency (1 Hz), strain (3%) and temperature (4 °C) for a certain time (4 h) under the gelation conditions used.
2.4.2. Morphological Evaluation
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Raw Material
3.2. Characterization of Hydrogels
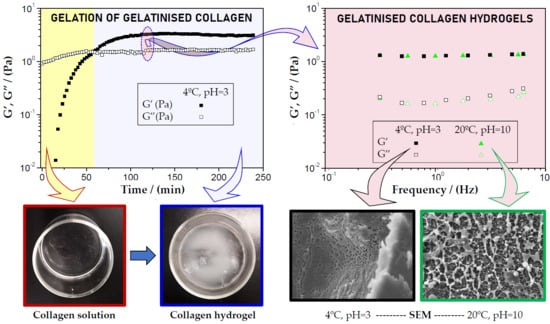
3.2.1. Influence of the Gelation Time
3.2.2. Influence of the pH and Gelation Temperature
3.2.3. Hydrogels Selected
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mertz, L. Tissue Engineering and Regenerative Medicine: The Promise, the Challenges, the Future. IEEE Pulse 2017, 8, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Reddi, A.H. Morphogenesis and tissue engineering of bone and cartilage: inductive signals, stem cells, and biomimetic biomaterials. Tissue Eng. 2000, 6, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006. [Google Scholar] [CrossRef] [PubMed]
- Lewin-Epstein, J. Polyvinyl sponge (Ivalon) as a scaffold for bone. Br. J. Oral Surg. 1964, 2, 115–119. [Google Scholar] [CrossRef]
- Vracko, R.; Benditt, E.P. Basal lamina: the scaffold for orderly cell replacement. Observations on regeneration of injured skeletal muscle fibers and capillaries. J. Cell Biol. 1972, 55, 406–419. [Google Scholar] [CrossRef]
- Li, W.J.; Cooper, J.A. Biomaterials for Tissue Engineering Applications; Burdick, J.A., Mauck, R.L., Eds.; Springer Vienna: Vienna, Austria, 2011; ISBN 978-3-7091-0384-5. [Google Scholar]
- Williams, D.F. On the nature of biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Rubio-Valle, J.F.; Jiménez-Rosado, M.; Guerrero, A.; Romero, A. Alternative processing methods of hybrid porous scaffolds based on gelatin and chitosan. J. Mech. Behav. Biomed. Mater. 2020, 102, 103472. [Google Scholar] [CrossRef]
- Xu, Q.; Gabbitas, B.; Matthews, S.; Zhang, D. The development of porous titanium products using slip casting. J. Mater. Process. Technol. 2013, 213, 1440–1446. [Google Scholar] [CrossRef]
- Korina, E.; Stoilova, O.; Manolova, N.; Rashkov, I. Multifunctional Hybrid Materials From Poly(3-Hydroxybutyrate), TiO2 Nanoparticles, and Chitosan Oligomers by Combining Electrospinning/Electrospraying and Impregnation. Macromol. Biosci. 2013, 13, 707–716. [Google Scholar] [CrossRef]
- University of Babylon Biomaterials generations. Available online: http://www.uobabylon.edu.iq/eprints/publication_12_1581_1707 (access on 10 March 2020).
- Dean, M.N.; Swanson, B.O.; Summers, A.P. Biomaterials: Properties, variation and evolution. Integr. Comp. Biol. 2009, 49, 15–20. [Google Scholar] [CrossRef]
- Etxabide, A.; Ribeiro, R.D.C.; Guerrero, P.; Ferreira, A.M.; Stafford, G.P.; Dalgarno, K.; de la Caba, K.; Gentile, P. Lactose-crosslinked fish gelatin-based porous scaffolds embedded with tetrahydrocurcumin for cartilage regeneration. Int. J. Biol. Macromol. 2018, 117, 199–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yunoki, S.; Ikoma, T.; Tanaka, J. Development of collagen condensation method to improve mechanical strength of tissue engineering scaffolds. Mater. Charact. 2010, 61, 907–911. [Google Scholar] [CrossRef]
- Ma, L.; Gao, C.; Mao, Z.; Zhou, J.; Shen, J.; Hu, X.; Han, C. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 2003, 24, 4833–4841. [Google Scholar] [CrossRef]
- Gaspar, A.; Moldovan, L.; Constantin, D.; Stanciuc, A.M.; Sarbu Boeti, P.M.; Efrimescu, I.C. Collagen-based scaffolds for skin tissue engineering. J. Med. Life 2011, 4, 172–177. [Google Scholar] [PubMed]
- Cui, M.; Liu, L.; Guo, N.; Su, R.; Ma, F. Preparation, cell compatibility and degradability of collagen-modified poly(lactic acid). Molecules 2015, 20, 595–607. [Google Scholar] [CrossRef] [Green Version]
- Gorczyca, G.; Tylingo, R.; Szweda, P.; Augustin, E.; Sadowska, M.; Milewski, S. Preparation and characterization of genipin cross-linked porous chitosan-collagen-gelatin scaffolds using chitosan-CO2 solution. Carbohydr. Polym. 2014, 102, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, X.; Cui, W.; Li, X.; Jin, Y. Electrospun Composite Mats of Poly[(D,L-lactide)-co-glycolide] and Collagen with High Porosity as Potential Scaffolds for Skin Tissue Engineering. Macromol. Mater. Eng. 2009, 294, 611–619. [Google Scholar] [CrossRef]
- Araki, K.; Halloran, J.W. New Freeze-Casting Technique for Ceramics with Sublimable Vehicles. J. Am. Ceram. Soc. 2004, 87, 1859–1863. [Google Scholar] [CrossRef] [Green Version]
- Perez-Puyana, V.M.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Highly porous protein-based 3D scaffolds with different collagen concentrates for potential application in tissue engineering. J. Appl. Polym. Sci. 2019, 136, 47954. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hochleitner, G.; Jungst, T.; Brown, T.D.; Hahn, K.; Moseke, C.; Jakob, F.; Dalton, P.D.; Groll, J. Additive manufacturing of scaffolds with sub-micron filaments via melt electrospinning writing. Biofabrication 2015, 7, 35002. [Google Scholar] [CrossRef] [PubMed]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Development of PVA/gelatin nanofibrous scaffolds for Tissue Engineering via electrospinning. Mater. Res. Express 2018, 5. [Google Scholar] [CrossRef]
- Yang, J.; Sun, X.; Zhang, Y.; Chen, Y. The application of natural polymer–based hydrogels in tissue engineering. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 273–307. [Google Scholar]
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A. Hydrogels in Medicine and Pharmacy: Fundamentals; Routledge Revivals; CRC Press: Boca Ratón, FL, USA, 2019; ISBN 9781000696943. [Google Scholar]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef]
- Shin, J.; Braun, P.V.; Lee, W. Fast response photonic crystal pH sensor based on templated photo-polymerized hydrogel inverse opal. Sensors Actuators B Chem. 2010, 150, 183–190. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels As Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Krsko, P.; McCann, T.E.; Thach, T.-T.; Laabs, T.L.; Geller, H.M.; Libera, M.R. Length-scale mediated adhesion and directed growth of neural cells by surface-patterned poly(ethylene glycol) hydrogels. Biomaterials 2009, 30, 721–729. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Klimek, K.; Ginalska, G. Proteins and Peptides as Important Modifiers of the Polymer Scaffolds for Tissue Engineering Applications—A Review. Polymers (Basel). 2020, 12, 844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peppas, N.A.; Khare, A.R. Preparation, structure and diffusional behavior of hydrogels in controlled release. Adv. Drug Deliv. Rev. 1993, 11, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Tachibana, A.; Furuta, Y.; Takeshima, H.; Tanabe, T.; Yamauchi, K. Fabrication of wool keratin sponge scaffolds for long-term cell cultivation. J. Biotechnol. 2002, 93, 165–170. [Google Scholar] [CrossRef]
- B Malafaya, P.P.; Pedro, A.J.; Peterbauer, A.; Gabriel, C.; Redl, H.; Reis, R.L. Chitosan particles agglomerated scaffolds for cartilage and osteochondral tissue engineering approaches with adipose tissue derived stem cells. J. Mater. Sci. Mater. Med. 2005, 16, 1077–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Allam, E.; Bottino, M.C.; Al-Shibani, N.; Jack Windsor, L. Collagen scaffolds: Tissue engineering and repair; Nova Science Publishers Inc: Hauppauge, NY, USA, 2012; ISBN 9781622576258. [Google Scholar]
- Perez-Puyana, V.; Ostos, F.J.; Lopez-Cornejo, P.; Romero, A.; Guerrero, A. Assessment of the denaturation of collagen protein concentrates using different techniques. Biol. Chem. 2019, 400(12), 1583–1591. [Google Scholar] [CrossRef]
- Yousefi, M.; Ariffin, F.; Huda, N. An alternative source of type I collagen based on by-product with higher thermal stability. Food Hydrocoll. 2017, 63, 372–382. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, L.; Sun, W.; Wang, Z.; Xu, J.; Ma, H. Isolation and characterization of collagen from the cartilage of Amur sturgeon ( Acipenser schrenckii ). Process Biochem. 2014, 49, 318–323. [Google Scholar] [CrossRef]
- AOAC, I. Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2005; ISBN 9780935584752. [Google Scholar]
- Markwell, M.A.K.; Haas, S.M.; Bieber, L.L.; Tolbert, N.E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef]
- Yang, H.; Xu, S.; Shen, L.; Liu, W.; Li, G. Changes in aggregation behavior of collagen molecules in solution with varying concentrations of acetic acid. Int. J. Biol. Macromol. 2016, 92, 581–586. [Google Scholar] [CrossRef]
- Sampath Kumar, N.S.; Nazeer, R.A.; Jai Ganesh, R. Functional properties of protein hydrolysates from different body parts of horse mackerel (Magalaspis cordyla) and croaker (Otolithes ruber). Med. J. Nutrition Metab. 2012, 5, 105–110. [Google Scholar] [CrossRef]
- Zinkovska, N.; Smilek, J.; Pekar, M. Gradient Hydrogels—The State of the Art in Preparation Methods. Polymers 2020, 12, 966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, A.M. Soy proteins. In Developments in food proteins; BJF Hudson: London and Englewood, NJ, USA, 1983; pp. 67–108. [Google Scholar]
- Veeruraj, A.; Arumugam, M.; Balasubramanian, T. Isolation and characterization of thermostable collagen from the marine eel-fish (Evenchelys macrura). Process Biochem. 2013, 48, 1592–1602. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Payne, K.J.; Veis, A. Fourier transform IR spectroscopy of collagen and gelatin solutions: deconvolution of the amide I band for conformational studies. Biopolymers 1988, 27, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.M.; Cordobés, F.; Jerez, A.; Guerrero, A. Influence of high pressure processing on the linear viscoelastic properties of egg yolk dispersions. Rheol. Acta 2007, 46, 731–740. [Google Scholar] [CrossRef]
- Bertasa, M.; Dodero, A.; Alloisio, M.; Vicini, S.; Riedo, C.; Sansonetti, A.; Scalarone, D.; Castellano, M. Agar gel strength: A correlation study between chemical composition and rheological properties. Eur. Polym. J. 2020, 123, 109442. [Google Scholar] [CrossRef]
- Yucel, T.; Cebe, P.; Kaplan, D.L. Vortex-Induced Injectable Silk Fibroin Hydrogels. Biophys. J. 2009, 97, 2044–2050. [Google Scholar] [CrossRef] [Green Version]
- Datta Chaudhuri, S.; Mandal, A.; Dey, A.; Chakrabarty, D. Tuning the swelling and rheological attributes of bentonite clay modified starch grafted polyacrylic acid based hydrogel. Appl. Clay Sci. 2020, 185, 105405. [Google Scholar] [CrossRef]
- Boruah, M.; Gogoi, P.; Manhar, A.K.; Khannam, M.; Mandal, M.; Dolui, S.K. Biocompatible carboxymethylcellulose-g-poly(acrylic acid)/OMMT nanocomposite hydrogel for in vitro release of vitamin B 12. RSC Adv. 2014, 4, 43865–43873. [Google Scholar] [CrossRef]
- Brazdaru, L.; Micutz, M.; Staicu, T.; Albu, M.; Sulea, D.; Leca, M. Structural and rheological properties of collagen hydrogels containing tannic acid and chlorhexidine digluconate intended for topical applications. Comptes Rendus Chim. 2015, 18, 160–169. [Google Scholar] [CrossRef]
- Cheng, L.; Cai, Z.; Ye, T.; Yu, X.; Chen, Z.; Yan, Y.; Qi, J.; Wang, L.; Liu, Z.; Cui, W.; et al. Injectable Polypeptide-Protein Hydrogels for Promoting Infected Wound Healing. Adv. Funct. Mater. 2020, 2001196. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Graulus, G.-J.; Keshari Samal, S.; Van Nieuwenhove, I.; Dubruel, P. 12 - Porous hydrogel biomedical foam scaffolds for tissue repair. In Biomedical Foams for Tissue Engineering Applications; Netti, P., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 335–390. ISBN 978-0-85709-696-8. [Google Scholar]
- Yannas, I. V Emerging rules for inducing organ regeneration. Biomaterials 2013, 34, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Khoshfetrat, A.B.; Khatami, N.; Ahmadian, M.; Rahbarghazi, R. Comparative study of collagen and gelatin in chitosan-based hydrogels for effective wound dressing: Physical properties and fibroblastic cell behavior. Biochem. Biophys. Res. Commun. 2019, 518, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Smart, L.E.; Moore, E.A. Solid State Chemistry: An Introduction, 2nd Edition; Taylor & Francis: London, UK, 1995; ISBN 9780748740680. [Google Scholar]
- Liu, Y.; Fan, D. Novel hyaluronic acid-tyrosine/collagen-based injectable hydrogels as soft filler for tissue engineering. Int. J. Biol. Macromol. 2019, 141, 700–712. [Google Scholar] [CrossRef]





| Gelation Time | γc (-) | G’1 (Pa) | tan δ1 (-) | η*1 (Pa·s) |
|---|---|---|---|---|
| 1 h | 0.48 ± 0.08 I | 0.53 ± 0.12 a | 0.52 ± 0.01 A | 0.27 ± 0.06 α |
| 2 h | 1.01 ± 0.05 II | 1.58 ± 0.42 b | 0.15 ± 0.03 B | 0.85 ± 0.26 β |
| 4 h | 1.01 ± 0.11 II | 2.55 ± 0.97 b | 0.14 ± 0.04 B | 1.74 ± 0.81 β |
| Temperature | pH | γc (-) | G’1 (Pa) | tan δ1 (-) | η*1 (Pa·s) |
|---|---|---|---|---|---|
| 4 °C | 3 | 1.01 ± 0.05 I | 1.58 ± 0.42 a | 0.15 ± 0.03 A | 0.85 ± 0.26 α |
| 5 | 1.01 ± 0.20 I | 0.22 ± 0.05 b | 2.69 ± 0.33 B | 0.25 ± 0.08 β | |
| 6.5 | 0.69 ± 0.10 II | 0.01 ± 0.01 c | 37.24 ± 3.09 C | 0.21 ± 0.03 β | |
| 8 | 1.01 ± 0.01 I | 0.78 ± 0.33 d | 0.62 ± 0.12 D | 0.52 ± 0.07 γ | |
| 10 | 1.01 ± 0.03 I | 0.82 ± 0.16 d | 0.68 ± 0.14 D | 0.34 ± 0.01 δ | |
| 20 °C | 3 | 0.42 ± 0.08 III | 1.01 ± 0.36 ad | 0.36 ± 0.05 E | 0.57 ± 0.17 γ |
| 5 | 0.48 ± 0.11 III | 0.29 ± 0.04 b | 0.75 ± 0.24 D | 0.15 ± 0.03 β | |
| 6.5 | 0.27 ± 0.05 IV | 0.55 ± 0.15 d | 0.58 ± 0.10 D | 0.31 ± 0.14 β | |
| 8 | 1.03 ± 0.12 I | 1.52 ± 0.45 a | 0.22 ± 0.02 F | 0.90 ± 0.25 α | |
| 10 | 1.01 ± 0.21 I | 1.38 ± 0.53 ad | 0.23 ± 0.04 F | 0.85 ± 0.19 α |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Fabrication and Characterization of Hydrogels Based on Gelatinised Collagen with Potential Application in Tissue Engineering. Polymers 2020, 12, 1146. https://doi.org/10.3390/polym12051146
Perez-Puyana V, Jiménez-Rosado M, Romero A, Guerrero A. Fabrication and Characterization of Hydrogels Based on Gelatinised Collagen with Potential Application in Tissue Engineering. Polymers. 2020; 12(5):1146. https://doi.org/10.3390/polym12051146
Chicago/Turabian StylePerez-Puyana, Victor, Mercedes Jiménez-Rosado, Alberto Romero, and Antonio Guerrero. 2020. "Fabrication and Characterization of Hydrogels Based on Gelatinised Collagen with Potential Application in Tissue Engineering" Polymers 12, no. 5: 1146. https://doi.org/10.3390/polym12051146
APA StylePerez-Puyana, V., Jiménez-Rosado, M., Romero, A., & Guerrero, A. (2020). Fabrication and Characterization of Hydrogels Based on Gelatinised Collagen with Potential Application in Tissue Engineering. Polymers, 12(5), 1146. https://doi.org/10.3390/polym12051146