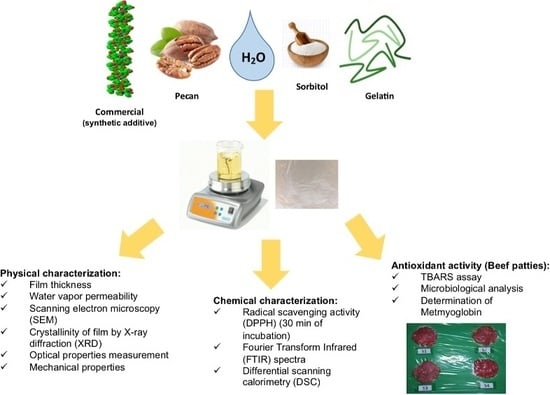
Characterization and Application of Gelatin Films with Pecan Walnut and Shell Extract (Carya illinoiensis)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample and Extracts Preparation
2.3. Film Preparation with PW and PWS Extracts
2.4. Chemical Analysis
2.4.1. Total Polyphenol Content (TPC) of PW and PWS Extract
2.4.2. Radical Scavenging Activity of PW and PWS
2.4.3. Radical Scavenging Activity of the Films Measured by DPPH
2.4.4. Fourier Transform Infrared (FTIR) Spectra
2.4.5. Differential Scanning Calorimetry (DSC)
2.5. Physical Analysis
2.5.1. Film Thickness
2.5.2. Water Vapor Permeability (WVP)
2.5.3. Scanning Electron Microscopy (SEM)
2.5.4. Crystallinity of Film by X-ray Diffraction (XRD)
2.5.5. Optical Properties Measurement
2.5.6. Mechanical Properties
2.6. Evaluation in Beef Patties
2.6.1. Preparation of Beef Patties
2.6.2. TBARS Assay
2.6.3. Microbiological Analysis
2.6.4. Determination of Metmyoglobin (MetMb)
2.6.5. Statistical Analysis
3. Results and Discussion
3.1. Total Polyphenol Content (TPC) and Radical Scavenging Activity (RSA) of PW and PWS
3.2. RSA of the Films by DPPH (2,2-diphenyl-1-picrylhydrazyl)
3.3. Fourier Transform Infrared (FTIR) Spectra
3.4. Differential Scanning Calorimetry (DSC)
3.5. Physical Analysis
3.5.1. Film Thickness
3.5.2. Water Vapor Permeability (WVP)
3.5.3. Scanning Electron Microscopy (SEM)
3.5.4. Crystallinity of Film by X-ray Diffraction (XRD)
3.5.5. Optical Properties Measurement
3.5.6. Mechanical Properties of the Films
3.5.7. Film Protection in Beef Patties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Abdelhedi, O.; Nasri, R.; Jridi, M.; Kchaou, H.; Nasreddine, B.; Karbowiak, T.; Debeaufort, F.; Nasri, M. Composite bioactive films based on smooth-hound viscera proteins and gelatin: Physicochemical characterization and antioxidant properties. Food Hydrocoll. 2018, 74, 176–186. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, J.; Chen, X.; Luo, M.; Liu, H.; Shao, P. Fabrication and characterization of multilayered kafirin/gelatin film with one-way water barrier property. Food Hydrocoll. 2018, 81, 159–168. [Google Scholar] [CrossRef]
- Pérez Córdoba, L.J.; Sobral, P.J.A. Physical and antioxidant properties of films based on gelatin, gelatin-chitosan or gelatin-sodium caseinate blends loaded with nanoemulsified active compounds. J. Food Eng. 2017, 213, 47–53. [Google Scholar] [CrossRef]
- Lee, K.Y.; Song, K. Bin Preparation and Characterization of an Olive Flounder (Paralichthys olivaceus) Skin Gelatin and Polylactic Acid Bilayer Film. J. Food Sci. 2017, 82, 706–710. [Google Scholar] [CrossRef]
- Benito-Peña, E.; González-Vallejo, V.; Rico-Yuste, A.; Barbosa-Pereira, L.; Cruz, J.M.; Bilbao, A.; Alvarez-Lorenzo, C.; Moreno-Bondi, M.C. Molecularly imprinted hydrogels as functional active packaging materials. Food Chem. 2016, 190, 487–494. [Google Scholar] [CrossRef]
- Etxabide, A.; Coma, V.; Guerrero, P.; Gardrat, C.; de la Caba, K. Effect of cross-linking in surface properties and antioxidant activity of gelatin films incorporated with a curcumin derivative. Food Hydrocoll. 2017, 66, 168–175. [Google Scholar] [CrossRef]
- Kim, H.; Beak, S.; Song, K. Bin Development of a hagfish skin gelatin film containing cinnamon bark essential oil. LWT Food Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Li, J.H.; Miao, J.; Wu, J.L.; Chen, S.F.; Zhang, Q.Q. Preparation and characterization of active gelatin based films incorporated with natural antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- dos Santos Garcia, V.A.; Borges, J.G.; Osiro, D.; Vanin, F.M.; de Carvalho, R.A. Orally disintegrating films based on gelatin and pregelatinized starch: New carriers of active compounds from acerola. Food Hydrocoll. 2020, 101, 105518. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, J.; Yu, H.; Zhang, J.; Yuan, Y.; Shen, X.; Li, C. The effects of EGCG on the mechanical, bioactivities, cross-linking and release properties of gelatin film. Food Chem. 2019, 271, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.L.; De Almeida, P.C.L.; Rodrigues, C.A.; De Battisti, L.F.F.; Costa, L.H.; Bastos, R.G.; De Oliveira, C.M.; Ferraz, V.P.; Moraes, A.L.L.; de Almeida Paula, H.A.; et al. Nutritional composition, fatty acid profile, phytochemistry and evaluation of the effects of Carya illinoinensis on diabetes. Int. J. Food Sci. Technol. 2019, 54, 2595–2603. [Google Scholar] [CrossRef]
- Delgado-Zamarreño, M.M.; Fernández-Prieto, C.; Bustamante-Rangel, M.; Pérez-Martín, L. Determination of tocopherols and sitosterols in seeds and nuts by QuEChERS-liquid chromatography. Food Chem. 2016, 192, 825–830. [Google Scholar]
- Villasante, J.; Girbal, M.; Meton, I.; Almajano, M.P. Effects of Pecan Nut (Carya illinoiensis) and Roselle Flower (Hibiscus sabdariffa) as Antioxidant and Antimicrobial Agents for Sardines (Sardina pilchardus). Molecules 2018, 24, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haddad, E.H. Health Effects of a Pecan [Carya illinoinensis (Wangenh.) K: Koch] Nut-Rich Diet; Department of Nutrition, School of Public Health, Loma Linda University: Loma Linda, CA, USA, 2011; ISBN 9780123756886. [Google Scholar]
- Villarreal-Lozoya, J.E.; Lombardini, L.; Cisneros-Zevallos, L. Phytochemical constituents and antioxidant capacity of different pecan [Carya illinoinensis (Wangenh.) K. Koch] cultivars. Food Chem. 2007, 102, 1241–1249. [Google Scholar] [CrossRef]
- Kellett, M.E.; Greenspan, P.; Gong, Y.; Pegg, R.B. Cellular evaluation of the antioxidant activity of U.S. Pecans [Carya illinoinensis (Wangenh.)K. Koch]. Food Chem. 2019, 293, 511–519. [Google Scholar] [CrossRef]
- Bolling, B.W.; Chen, C.-Y.O.; McKay, D.L.; Blumberg, J.B. Tree nut phytochemicals: Composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr. Res. Rev. 2011, 24, 244–275. [Google Scholar] [CrossRef] [Green Version]
- Alasalvar, C.; Bolling, B.W. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br. J. Nutr. 2015, 113, S68–S78. [Google Scholar] [CrossRef]
- Pirayesh, H.; Khazaeian, A.; Tabarsa, T. The potential for using walnut (Juglans regia L.) shell as a raw material for wood based particleboard manufacturing. Compos. Part B Eng. 2012, 43, 3276–3280. [Google Scholar] [CrossRef]
- De La Rosa, L.A.; Alvarez-Parrilla, E.; Shahidi, F. Phenolic compounds and antioxidant activity of kernels and shells of Mexican pecan (Carya illinoinensis). J. Agric. Food Chem. 2011, 59, 152–162. [Google Scholar] [CrossRef]
- do Prado, A.C.P.; Monalise Aragão, A.; Fett, R.; Block, J.M. Antioxidant Properties of Pecan Nut [Carya illinoinensis (Wangenh.) C. Koch] Shell Infusion. Grasas y Aceites 2009, 60, 330–335. [Google Scholar] [CrossRef] [Green Version]
- Hilbig, J.; de Britto Policarpi, P.; de Souza Grinevicius, V.M.A.; Mota, N.S.R.S.; Toaldo, I.M.; Luiz, M.T.B.; Pedrosa, R.C.; Block, J.M. Aqueous extract from pecan nut [Carya illinoinensis (Wangenh) C. Koch] shell show activity against breast cancer cell line MCF-7 and Ehrlich ascites tumor in Balb-C mice. J. Ethnopharmacol. 2018, 211, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Hilbig, J.; Alves, V.R.; Müller, C.M.O.; Micke, G.A.; Vitali, L.; Pedrosa, R.C.; Block, J.M. Ultrasonic-assisted extraction combined with sample preparation and analysis using LC-ESI-MS/MS allowed the identification of 24 new phenolic compounds in pecan nut shell [Carya illinoinensis (Wangenh) C. Koch] extracts. Food Res. Int. 2018, 106, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.; Kim, J.; Chung, J. Production of granular activated carbon from food-processing wastes (walnut shells and jujube seeds) and its adsorptive properties. J. Air Waste Manag. Assoc. 2014, 64, 879–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosca, F.; Hidalgo, G.; Villasante, J.; Almajano, M. Continuous or Batch Solid-Liquid Extraction of Antioxidant Compounds from Seeds of Sterculia apetala Plant and Kinetic Release Study. Molecules 2018, 23, 1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallego, M.G.; Gordon, M.H.; Segovia, F.J.; Skowyra, M. Antioxidant Properties of Three Aromatic Herbs (Rosemary, Thyme and Lavender) in Oil-in-Water Emulsions. JAOCS J. Am. Oil Chem. Soc. 2013, 1559–1568. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Antioxidative activity and properties of fish skin gelatin films incorporated with BHT and a-tocopherol. Food Hydrocoll. 2008, 22, 449–458. [Google Scholar] [CrossRef]
- Staroszczyk, H.; Sztuka, K.; Wolska, J.; Wojtasz-Paja̧k, A.; Kołodziejska, I. Interactions of fish gelatin and chitosan in uncrosslinked and crosslinked with EDC films: FT-IR study. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2014, 117, 707–712. [Google Scholar] [CrossRef]
- Ramis, X.; Salla, J.M.; Cadenato, A.; Morancho, J.M. Simulation of isothermal cure of a powder coating non-isothermal DSC experiments. J. Therm. Anal. Calorim. 2003, 72, 707–718. [Google Scholar] [CrossRef]
- Shiku, Y.; Hamaguchi, P.Y.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Effect of surimi quality on properties of edible films based on Alaska pollack. Food Chem. 2004, 86, 493–499. [Google Scholar] [CrossRef]
- Soo, P.Y.; Sarbon, N.M. Preparation and characterization of edible chicken skin gelatin film incorporated with rice flour. Food Packag. Shelf Life 2018, 15, 1–8. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Whey protein edible films modified with almond and walnut oils. Food Hydrocoll. 2016, 52, 78–86. [Google Scholar] [CrossRef]
- International Standard. International Standard ISO 527-1, Plastics, Determination of Tensile Properties; ISO Copyright Office: Geneva, Switzerland, 2012; Volume 2012, ISBN 9782832223734. [Google Scholar]
- Ouerfelli, M.; Villasante, J.; Ben Kaâb, L.B.; Almajano, M.P. Effect of neem (Azadirachta indica L.) on lipid oxidation in raw chilled beef patties. Antioxidants 2019, 8, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Córdova, M.A.; Sánchez, E.; Muñoz-Márquez, E.; Ojeda-Barrios, D.L.; Soto-Parra, J.M.; Preciado-Rangel, P. Phytochemical composition and antioxidant capacity in Mexican pecan nut. Emirates J. Food Agric. 2017, 29, 346–350. [Google Scholar] [CrossRef] [Green Version]
- Navikaite-Snipaitiene, V.; Ivanauskas, L.; Jakstas, V.; Rüegg, N.; Rutkaite, R.; Wolfram, E.; Yildirim, S. Development of antioxidant food packaging materials containing eugenol for extending display life of fresh beef. Meat Sci. 2018, 145, 9–15. [Google Scholar] [CrossRef]
- Moghaddas Kia, E.; Ghaderzadeh, S.; Mojaddar Langroodi, A.; Ghasempour, Z.; Ehsani, A. Red beet extract usage in gelatin/gellan based gummy candy formulation introducing Salix aegyptiaca distillate as a flavouring agent. J. Food Sci. Technol. 2020, 84, 12–20. [Google Scholar] [CrossRef]
- Villasante, J.; Pérez-Carrillo, E.; Heredia-Olea, E.; Metón, I.; Almajano, M.P. In Vitro Antioxidant Activity Optimization of Nut Shell (Carya illinoinensis) by Extrusion Using Response Surface Methods. Biomolecules 2019, 9, 883. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, G.D.; Gómez-Coca, R.B.; del Carmen Pérez-Camino, M.; Moreda, W.; Barrera-Arellano, D. Chemical Characterization of Major and Minor Compounds of Nut Oils: Almond, Hazelnut, and Pecan Nut. J. Chem. 2017, 2017, 2609549. [Google Scholar] [CrossRef] [Green Version]
- Cano, A.; Andres, M.; Chiralt, A.; González-Martinez, C. Use of tannins to enhance the functional properties of protein based films. Food Hydrocoll. 2020, 100. [Google Scholar] [CrossRef]
- Fan, Y.; Yi, J.; Hua, X.; Zhang, Y.; Yang, R. Preparation and characterization of gellan gum microspheres containing a cold-adapted β-galactosidase from Rahnella sp. R3. Carbohydr. Polym. 2017, 162, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Payne, K.J.; Veis, A. Fourier transform ir spectroscopy of collagen and gelatin solutions: Deconvolution of the amide I band for conformational studies. Biopolymers 1988, 27, 1749–1760. [Google Scholar] [CrossRef]
- Uranga, J.; Etxabide, A.; Guerrero, P.; de la Caba, K. Development of active fish gelatin films with anthocyanins by compression molding. Food Hydrocoll. 2018, 84, 313–320. [Google Scholar] [CrossRef]
- Harini, K.; Chandra Mohan, C.; Ramya, K.; Karthikeyan, S.; Sukumar, M. Effect of Punica granatum peel extracts on antimicrobial properties in Walnut shell cellulose reinforced Bio-thermoplastic starch films from cashew nut shells. Carbohydr. Polym. 2018, 184, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Boumail, A.; Salmieri, S.; Klimas, E.; Tawema, P.O.; Bouchard, J.; Lacroix, M. Characterization of trilayer antimicrobial diffusion films (ADFs) based on methylcellulose-polycaprolactone composites. J. Agric. Food Chem. 2013, 61, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Hoque, S.; Benjakul, S.; Prodpran, T.; Songtipya, P. International Journal of Biological Macromolecules Properties of blend film based on cuttlefish (Sepia pharaonis) skin gelatin and mungbean protein isolate. Int. J. Biol. Macromol. 2011, 49, 663–673. [Google Scholar] [CrossRef]
- Wang, W.; Wang, K.; Xiao, J.; Liu, Y.; Zhao, Y.; Liu, A. Performance of high amylose starch-composited gelatin films influenced by gelatinization and concentration. Int. J. Biol. Macromol. 2017, 94, 258–265. [Google Scholar] [CrossRef]
- Riaz, A.; Lagnika, C.; Abdin, M.; Hashim, M.M.; Ahmed, W. Preparation and Characterization of Chitosan/Gelatin based Active Food Packaging Films Containing Apple Peel Nanoparticles. Carbohydr. Polym. 2020, 85, 50–56. [Google Scholar] [CrossRef]
- Zhang, H.; He, P.; Kang, H.; Li, X. Antioxidant and antimicrobial effects of edible coating based on chitosan and bamboo vinegar in ready to cook pork chops. LWT 2018, 93, 470–476. [Google Scholar] [CrossRef]
- Trejo, V.; Aragon, N.; Miranda, P. Estimación de la permeabilidad al vapor de agua en películas a base de quitosán. Rev. la Sociadad Química México 2001, 45, 1–5. [Google Scholar]
- Salvador, A.A.; Podestá, R.; Block, J.M.; Ferreira, S.R.S. Increasing the value of pecan nut [Carya illinoinensis (Wangenh) C. Koch] cake by means of oil extraction and antioxidant activity evaluation. J. Supercrit. Fluids 2016, 116, 215–222. [Google Scholar] [CrossRef]
- Halimatul, M.J.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Ilyas, R.A. Water absorption and water solubility properties of sago starch biopolymer composite films filled with sugar palm particles. Polimery/Polymers 2019, 64, 596–604. [Google Scholar] [CrossRef] [Green Version]
- Fakhreddin, S.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Development of bioactive fish gelatin/chitosan nanoparticles composite films with antimicrobial properties. Food Chem. 2016, 194, 1266–1274. [Google Scholar] [CrossRef]
- Acosta, S.; Jiménez, A.; Cháfer, M.; González-Martínez, C.; Chiralt, A. Physical properties and stability of starch-gelatin based films as affected by the addition of esters of fatty acids. Food Hydrocoll. 2015, 49, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Jridi, M.; Hajji, S.; Ayed, H.B.; Lassoued, I.; Mbarek, A.; Kammoun, M.; Souissi, N.; Nasri, M. Physical, structural, antioxidant and antimicrobial properties of gelatin-chitosan composite edible films. Int. J. Biol. Macromol. 2014, 67, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.M.B.; Tomé, L.C.; Garcia, H.; Brandão, L.; Mendes, A.M.; Marrucho, I.M. Effect of natural and synthetic antioxidants incorporation on the gas permeation properties of poly(lactic acid) films. J. Food Eng. 2013, 116, 562–571. [Google Scholar]
- Pastor, C.; Sánchez-González, L.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical and antifungal properties of hydroxypropylmethylcellulose based films containing propolis as affected by moisture content. Carbohydr. Polym. 2010, 82, 1174–1183. [Google Scholar] [CrossRef]
- Chang-Bravo, L.; López-Córdoba, A.; Martino, M. Biopolymeric matrices made of carrageenan and corn starch for the antioxidant extracts delivery of Cuban red propolis and yerba mate. React. Funct. Polym. 2014, 85, 11–19. [Google Scholar] [CrossRef]
- Teodoro, A.P.; Mali, S.; Romero, N.; De Carvalho, G.M. Cassava starch films containing acetylated starch nanoparticles as reinforcement: Physical and mechanical characterization. Carbohydr. Polym. 2015, 126, 9–16. [Google Scholar] [CrossRef]
- Iahnke, A.O.E.S.; Costa, T.M.H.; De Oliveira Rios, A.; Flôres, S.H. Antioxidant films based on gelatin capsules and minimally processed beet root (Beta vulgaris L. var. Conditiva) residues. J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar] [CrossRef]
- Kojić-Prodić, B. A century of X-ray crystallography and 2014 international year of X-ray crystallography. Maced. J. Chem. Chem. Eng. 2015, 34, 19. [Google Scholar] [CrossRef] [Green Version]
- Rivero, S.; García, M.A.; Pinotti, A. Correlations between structural, barrier, thermal and mechanical properties of plasticized gelatin films. Innov. Food Sci. Emerg. Technol. 2010, 11, 369–375. [Google Scholar] [CrossRef]
- Yakimets, I.; Wellner, N.; Smith, A.C.; Wilson, R.H.; Farhat, I.; Mitchell, J. Mechanical properties with respect to water content of gelatin films in glassy state. Polymer (Guildf.) 2005, 46, 12577–12585. [Google Scholar] [CrossRef]
- Pępczyńska, M.; Díaz-Calderón, P.; Quero, F.; Matiacevich, S.; Char, C.; Enrione, J. Interaction and fragility study in salmon gelatin-oligosaccharide composite films at low moisture conditions. Food Hydrocoll. 2019, 97. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Guerrero, P.; de la Caba, K.; Benjakul, S.; Prodpran, T. Properties of fish gelatin films containing epigallocatechin gallate fabricated by thermo-compression molding. Food Hydrocoll. 2019, 97, 105236. [Google Scholar] [CrossRef]
- Lerma-Herrera, M.A.; Núñez-Gastélum, J.A.; Ascacio-Valdés, J.; Aguilar, C.N.; Rodrigo-García, J.; Díaz-Sánchez, A.G.; Alvarez-Parrilla, E.; de la Rosa, L.A. Estimation of the Mean Degree of Polymerization of Condensed Tannins from the Kernel and Shell of Carya illinoinensis by HPLC/MS and Spectrophotometric Methods. Food Anal. Methods 2017, 10, 3023–3031. [Google Scholar] [CrossRef]
- Pereda, M.; Aranguren, M.I.; Marcovich s, N.E. Caseinate films modified with tung oil. Food Hydrocoll. 2010, 24, 800–808. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S. Fatty acids and their sucrose esters affect the properties of fish skin gelatin based film. Eur. Food Res. Technol. 2006, 222, 650–657. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Grossmann, M.V.E.; Yamashita, F.; Pineda, E.A.G. Antimicrobial, Mechanical, and Barrier Properties of Cassava Starch-Chitosan Films Incorporated with Oregano Essential Oil. Int. Food Res. J. 2010, 17, 63–69. [Google Scholar] [CrossRef]
- Ge, L.; Zhu, M.; Li, X.; Xu, Y.; Ma, X.; Shi, R.; Li, D.; Mu, C. Development of active rosmarinic acid-gelatin biodegradable films with antioxidant and long-term antibacterial activities. Food Hydrocoll. 2018, 83, 308–316. [Google Scholar] [CrossRef]
- Putsakum, G.; Lee, D.S. The properties of gelatin film—Neem extract and its effectiveness for preserving minced beef. Packag. Technol. Sci. 2018, 31, 1–10. [Google Scholar] [CrossRef]
- Bonilla, J.; Poloni, T.; Lourenço, R.V.; Sobral, P.J.A. Food Bioscience Antioxidant potential of eugenol and ginger essential oils with gelatin/chitosan films. Food Biosci. 2018, 23, 107–114. [Google Scholar] [CrossRef]
- Theerawitayaart, W.; Prodpran, T.; Benjakul, S.; Sookchoo, P. Propierties of films from fish gelatin prepared by molecular modification and direct addition of oxidized linoleic acid. Food Hydrocoll. 2018, 88, 291–300. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Edible films and coatings to prevent the detrimental effect of oxygen on food quality: Possibilities and limitations. J. Food Eng. 2012, 110, 208–213. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Ahn, D.U.; Nam, K.C.; Jo, C. Factors affecting cooked chicken meat flavour: A review. Worlds. Poult. Sci. J. 2013, 69, 515–526. [Google Scholar] [CrossRef] [Green Version]
- Steele, K.S.; Weber, M.J.; Boyle, E.A.E.; Hunt, M.C.; Lobaton-Sulabo, A.S.; Cundith, C.; Hiebert, Y.H.; Abrolat, K.A.; Attey, J.M.; Clark, S.D.; et al. Shelf life of fresh meat products under LED or fluorescent lighting. Meat Sci. 2016, 117, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Brychcy, E.; Król, Ż.; Kulig, D.; Jarmoluk, A. The effect of carrageenan and gelatine hydrosols incorporated with acidic electrolysed water on surface microbiota and quality changes on pork meat. Int. J. Food Sci. Technol. 2016, 51, 1618–1629. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Kang, H. Chitosan coatings incorporated with free or nano-encapsulated Paulownia Tomentosa essential oil to improve shelf-life of ready-to-cook pork chops. Lwt 2019, 116, 108580. [Google Scholar] [CrossRef]
- Tammineni, N.; Ünlü, G.; Rasco, B.; Powers, J.; Sablani, S.; Nindo, C. Trout-Skin Gelatin based Edible Films Containing Phenolic Antioxidants: Effect on Physical Properties and Oxidative Stability of Cod-Liver Oil Model Food. J. Food Sci. 2012, 77, 1–6. [Google Scholar] [CrossRef]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Fernández-Prior, Á.; Fernández-Bolaños, J. Effect of edible pectin-fish gelatin films containing the olive antioxidants hydroxytyrosol and 3,4-dihydroxyphenylglycol on beef meat during refrigerated storage. Meat Sci. 2019, 148, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Umaraw, P.; Verma, A.K. Comprehensive review on application of edible film on meat and meat products: An eco-friendly approach. Crit. Rev. Food Sci. Nutr. 2017, 57, 1270–1279. [Google Scholar] [CrossRef]
- Lagos, M.J.B.; do Amaral Sobral, P.J. Application of active films with natural extract for beef hamburger preservation. Ciênc. Rural 2019, 49, 1–9. [Google Scholar] [CrossRef]
- Bentayeb, K.; Rubio, C.; Batlle, R.; Nerín, C. Direct determination of carnosic acid in a new active packaging based on natural extract of rosemary. Anal. Bioanal. Chem. 2007, 389, 1989–1996. [Google Scholar] [CrossRef] [PubMed]







| Sample | TPC [mg GAE/g DW] | DPPH [µmol TE/g DW] |
|---|---|---|
| PW | 20.55 ± 0.03 a | 37.63 ± 1.08 a |
| PWS | 72.96 ± 0.02 b | 205.12± 3.00 b |
| Peak (CM−1) | n. | Functional Group | Bond | Control Film | 3% PW Film | 9% PW Film | 15% PW Film | 3% PWS Film | 1% Commercial Film | 0.1% Commercial Film |
|---|---|---|---|---|---|---|---|---|---|---|
| 3289.11–3281.09 | 1 | Amines/Amides/-OH | N-H/O-H | 81.09% | 93.48% | 93.59% | 95.67% | -- | 88.07% | 93.73% |
| 3007.26–3007.59 | 2 | Aromatic | C–H | -- | -- | 93.90% | 93.2% | -- | -- | -- |
| 2934.24–2923.27 | 3 | Alquilo | –C–H | 91.09% | 91.84% | 68.08% | 71.25% | -- | 92.82% | 95.14% |
| 1743.74–1743.29 | 4 | Acid carboxylic | C=O | -- | 95.32% | 66.68% | 72.24% | -- | -- | -- |
| 1630.86–1645.51 | 5 | Carbonyl | C=O | 62.12% | 86% | 89.37% | 85.49% | 98.37% | 80.55% | 71.14% |
| 1548.37–1541.41 | 6 | Amines blending | N–H | 67.68% | 87.51% | 90.89% | 88.05% | 98.15% | 83.08% | 83.66% |
| Sample | Thickness (mm) |
|---|---|
| Control | 0.032 ± 0.01 d |
| 15% PW | 0.150 ± 0.02 a |
| 9% PW | 0.079 ± 0.00 b |
| 3% PW | 0.047 ± 0.00 d |
| 3% PWS | 0.076 ± 0.00 bc |
| 1% Commercial | 0.051 ± 0.00 cd |
| 0.1% Commercial | 0.047 ± 0.01 d |
| Film | CIE L* | CIE a* | CIE b* | CIE ΔE |
|---|---|---|---|---|
| Control | 93.75 ± 0.23 a | −0.43 ± 0.34 de | 3.55 ± 0.43 e | 0.00 ± 0.00 e |
| 3% PW | 91.72 ± 0.92 ab | 0.01 ± 0.16 de | 7.48 ± 1.82 cde | 4.78 ± 2.04 cde |
| 9% PW | 89.20 ± 0.87 bcd | 0.33 ± 0.16 de | 12.77 ± 1.67 b | 10.32 ± 1.89 b |
| 15% PW | 84.03 ± 0.60 e | 2.49 ± 0.35 ab | 18.51 ± 0.66 a | 18.08 ± 0.93 a |
| 3% PWS | 86.71 ± 0.44 de | 3.19 ± 0.25 a | 12.21 ± 0.41 bc | 11.73 ± 0.65 b |
| 1% Commercial | 93.61 ± 0.21 a | –0.61 ± 0.23 e | 4.39 ± 0.45 de | 0.87 ± 0.32 de |
| 0.1% Commercial | 93.62 ± 0.07 a | –0.54 ± 0.02 e | 4.66 ± 0.12 e | 1.13 ± 0.13 e |
| Sample | YM (MPa) | UTS (MPa) | EB (%) |
|---|---|---|---|
| Control | 797.92 ± 250.90 ab | 39.93 ± 13.56 ab | 6.39 ± 0.12 d |
| 3% PW | 615.47 ± 172.79 abc | 37.51 ± 2.70 ab | 8.68 ± 2.57 cd |
| 9% PW | 358.96 ± 16.24 bcd | 16.74 ± 2.90 bc | 25.70 ± 1.99 b |
| 15% PW | 96.70 ± 17.65 d | 5.95 ± 1.05 c | 31.19 ± 4.28 bc |
| 3% PWS | 513.99 ± 34.01 abcd | 21.84 ± 1.83 abc | 6.39 ± 0.03 d |
| 1% Commercial | 883.24 ± 16.84 a | 43.79 ± 3.68 a | 7.17 ± 1.39 d |
| 0.1% Commercial | 964.25 ± 232.44 a | 48.06 ± 19.76 a | 8.29 ± 3.76 cd |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villasante, J.; Martin-Lujano, A.; Almajano, M.P. Characterization and Application of Gelatin Films with Pecan Walnut and Shell Extract (Carya illinoiensis). Polymers 2020, 12, 1424. https://doi.org/10.3390/polym12061424
Villasante J, Martin-Lujano A, Almajano MP. Characterization and Application of Gelatin Films with Pecan Walnut and Shell Extract (Carya illinoiensis). Polymers. 2020; 12(6):1424. https://doi.org/10.3390/polym12061424
Chicago/Turabian StyleVillasante, Juliana, Anna Martin-Lujano, and María Pilar Almajano. 2020. "Characterization and Application of Gelatin Films with Pecan Walnut and Shell Extract (Carya illinoiensis)" Polymers 12, no. 6: 1424. https://doi.org/10.3390/polym12061424
APA StyleVillasante, J., Martin-Lujano, A., & Almajano, M. P. (2020). Characterization and Application of Gelatin Films with Pecan Walnut and Shell Extract (Carya illinoiensis). Polymers, 12(6), 1424. https://doi.org/10.3390/polym12061424