Chitosan Grafted with Biobased 5-Hydroxymethyl-Furfural as Adsorbent for Copper and Cadmium Ions Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Synthesis of CS-HMF Derivatives
2.2. Characterization Techniques
2.3. Adsorption/desorption Experiments
2.3.1. Adsorption Experiments
Effect of pH
Effect of Contact Time
Effect of Initial Ion Concentration and Temperature
2.3.2. Desorption and Reuse Experiments
3. Results and Discussion
3.1. Evaluation of Characterization Techniques: SEM, XRD, TGA and FTIR
3.2. Adsorption Evaluation
3.2.1. Effect of pH
3.2.2. Effect of Contact time
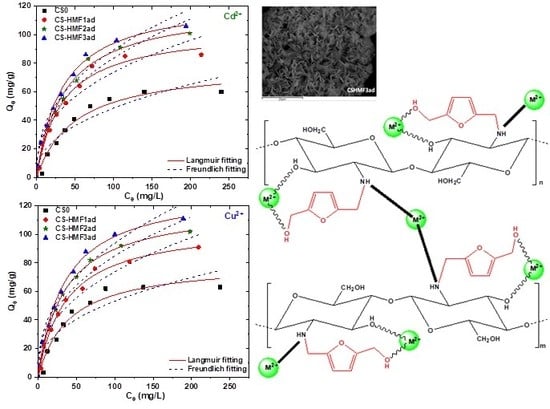
3.2.3. Effect of Initial Ion Concentration and Temperature
3.3. Desorption/Reuse Cycles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Gkika, A.D.; Liakos, V.E.; Vordos, N.; Kontogoulidou, C.; Magafas, L.; Bikiaris, N.D.; Bandekas, V.D.; Mitropoulos, C.A.; Kyzas, Z.G. Cost estimation of polymeric adsorbents. Polymers 2019, 11, 925. [Google Scholar] [CrossRef] [Green Version]
- Kyzas, G.Z.; Kostoglou, M.; Lazaridis, N.K.; Lambropoulou, D.A.; Bikiaris, D.N. Environmental friendly technology for the removal of pharmaceutical contaminants from wastewaters using modified chitosan adsorbents. Chem. Eng. J. 2013, 222, 248–258. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, W. Adsorption of Pb2+ from aqueous solutions using novel functionalized corncobs via atom transfer radical polymerization. Polymers 2019, 11, 1715. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.D.; Vu, T.N.; Nguyen, H.L.; Le, P.H.P.; Hoang, T.S. Adsorptive removal of antibiotic ciprofloxacin from aqueous solution using protein-modified nanosilica. Polymers 2020, 12, 57. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Jamil, S.N.A.M.; Choong, T.S.Y.; Abdullah, A.H.; Mastuli, M.S.; Othman, N.; Jiman, N.N. Green flexible polyurethane foam as a potent support for Fe-Si adsorbent. Polymers 2019, 11, 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maponya, T.C.; Ramohlola, K.E.; Kera, N.H.; Modibane, K.D.; Maity, A.; Katata-Seru, L.M.; Hato, M.J. Influence of magnetic nanoparticles on modified polypyrrole/m-phenylediamine for adsorption of Cr(VI) from aqueous solution. Polymers 2020, 12, 679. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.; Yang, Z.; Huang, L.; He, Y.; Wang, H.; Zhang, L. Macroscopic poly schiff base-coated bacteria cellulose with high adsorption performance. Polymers 2020, 12, 714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaipulizan, N.S.; Jamil, S.N.A.M.; Kamaruzaman, S.; Subri, N.N.S.; Adeyi, A.A.; Abdullah, A.H.; Abdullah, L.C. Preparation of ethylene glycol dimethacrylate (EGDMA)-based terpolymer as potential sorbents for pharmaceuticals adsorption. Polymers 2020, 12, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.A.; Momina; Siddiqui, M.R.; Otero, M.; Alshareef, S.A.; Rafatullah, M. Removal of rhodamine b from water using a solvent impregnated polymeric dowex 5wx8 resin: Statistical optimization and batch adsorption studies. Polymers 2020, 12, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudre, G.; Siband, E.; Gallas, B.; Cousin, F.; Hourdet, D.; Tran, Y. Responsive adsorption of N-isopropylacrylamide based copolymers on polymer brushes. Polymers 2020, 12, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Yang, Z.Y.; Cheng, X.W.; Tang, R.C.; Qiao, Y.F. Adsorption, antibacterial and antioxidant properties of Tannic Acid on silk fiber. Polymers 2019, 11, 970. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Xia, T.; Pei, M.; Du, Y.; Wang, L. Bentonite modified by allylamine polymer for adsorption of amido black 10B. Polymers 2019, 11, 502. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.; Sohn, J.S.; Kim, H.K.; Ryu, Y.; Cha, S.W. Effects of gas adsorption on the mechanical properties of amorphous polymer. Polymers 2019, 11, 817. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhao, J.; Wang, S.; Zhang, L.; Zhang, B. Efficient and selective adsorption of gold ions from wastewater with polyaniline modified by trimethyl phosphate: Adsorption mechanism and application. Polymers 2019, 11, 652. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Diao, K.; Tan, X.; Lei, F.; Jiang, J.; Goodman, B.A.; Ma, Y.; Liu, S. Mechanisms of adsorption of heavy metal cations from waters by an amino bio-based resin derived from Rosin. Polymers 2019, 11, 969. [Google Scholar] [CrossRef] [Green Version]
- Kong, W.; Chang, M.; Zhang, C.; Liu, X.; He, B.; Ren, J. Preparation of xylan-g-/P(AA-co-AM)/GO nanocomposite hydrogel and its adsorption for heavy metal ions. Polymers 2019, 11, 621. [Google Scholar] [CrossRef] [Green Version]
- Sims, R.A.; Harmer, S.L.; Quinton, J.S. The role of physisorption and chemisorption in the oscillatory adsorption of organosilanes on aluminium oxide. Polymers 2019, 11, 410. [Google Scholar] [CrossRef] [Green Version]
- Othman, N.A.F.; Selambakkannu, S.; Abdullah, T.A.T.; Hoshina, H.; Sattayaporn, S.; Seko, N. Selectivity of copper by amine-based ion recognition polymer adsorbent with different aliphatic amines. Polymers 2019, 11, 1994. [Google Scholar] [CrossRef] [Green Version]
- Clark, G.L.; Smith, A.F. X-ray diffraction studies of chitin, chitosan, and derivatives. J. Phys. Chem. 1936, 40, 863–879. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Natural Chelating Polymers: Alginic Acid, Chitin, and Chitosan; Pergamon Press: Oxford, UK, 1973; Volume 55. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Lamb, G.F. The chemical constitution of chitin. Science 1916, 44, 866–868. [Google Scholar]
- Irvine, J.C. LXXII.—A polarimetric method of identifying chitin. J. Chem. Soc. Trans. 1909, 95, 564–570. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, W.; Liu, P.; Cheng, Q.; Tahirou, T.; Gu, W.; Li, B. Chitosan modification and pharmaceutical/biomedical applications. Mar. Drugs 2010, 8, 1962–1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Z.S.; Xu, Y.L.; Zou, X.T.; Xu, Z.R. Chitosan nanoparticles act as an adjuvant to promote both Th1 and Th2 immune responses induced by ovalbumin in mice. Mar. Drugs 2011, 9, 1038–1055. [Google Scholar] [CrossRef]
- Wen, Z.S.; Liu, L.J.; Qu, Y.L.; OuYang, X.K.; Yang, L.Y.; Xu, Z.R. Chitosan nanoparticles attenuate hydrogen peroxide-induced stress injuryin mouse macrophage RAW264.7 cells. Mar. Drugs 2013, 11, 3582–3600. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, J.; Kim, S.K. Chitosan composites for bone tissue engineering—An overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, J.; Bhatnagar, I.; Kim, S.K. Chitosan-Alginate biocomposite containing fucoidan for bone tissue engineering. Mar. Drugs 2014, 12, 300–316. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Rodríguez-Amado, I.; Montemayor, M.I.; Fraguas, J.; Del González, M.P.; Murado, M.A. Chondroitin sulfate, hyaluronic acid and chitin/chitosan production using marine waste sources: Characteristics, applications and eco-friendly processes: A review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [CrossRef] [Green Version]
- Tsai, Z.T.; Tsai, F.Y.; Yang, W.C.; Wang, J.F.; Liu, C.L.; Shen, C.R.; Yen, T.C. Preparation and characterization of ferrofluid stabilized with biocompatible chitosan and dextran sulfate hybrid biopolymer as a potential magnetic resonance imaging (MRI) T2 contrast agent. Mar. Drugs 2012, 10, 2403–2414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muzzarelli, R.A.A. Biomedical exploitation of chitin and chitosan via mechano-chemical disassembly, electrospinning, dissolution in imidazolium ionic liquids, and supercritical drying. Mar. Drugs 2011, 9, 1510–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Shan, C.L.; Zhou, Q.; Fang, Y.; Wang, Y.L.; Xu, F.; Han, L.R.; Ibrahim, M.; Guo, L.B.; Xie, G.L.; et al. Synthesis, characterization, and antibacterial activity of cross-linked chitosan-glutaraldehyde. Mar. Drugs 2013, 11, 1534–1552. [Google Scholar] [CrossRef] [Green Version]
- Korkiatithaweechai, S.; Umsarika, P.; Praphairaksit, N.; Muangsin, N. Controlled release of diclofenac from matrix polymer of chitosan and oxidized konjac glucomannan. Mar. Drugs 2011, 9, 1649–1663. [Google Scholar] [CrossRef]
- Guo, L.; Liu, G.; Hong, R.Y.; Li, H.Z. Preparation and characterization of chitosan poly(acrylic acid) magnetic microspheres. Mar. Drugs 2010, 8, 2212–2222. [Google Scholar] [CrossRef]
- Cheng, M.; Gao, X.; Wang, Y.; Chen, H.; He, B.; Xu, H.; Li, Y.; Han, J.; Zhang, Z. Synthesis of glycyrrhetinic acid-modified chitosan 5-fluorouracil nanoparticles and its inhibition of liver cancer characteristics in vitro and in vivo. Mar. Drugs 2013, 11, 3517–3536. [Google Scholar] [CrossRef]
- Chen, J.K.; Yeh, C.H.; Wang, L.C.; Liou, T.H.; Shen, C.R.; Liu, C.L. Chitosan, the marine functional food, is a potent adsorbent of humic acid. Mar. Drugs 2011, 9, 2488–2498. [Google Scholar] [CrossRef]
- Maia, L.F.O.; Santos, M.S.; Andrade, T.G.; Hott, R.D.C.; Faria, M.C.D.S.; Oliveira, L.C.A.; Pereira, M.C.; Rodrigues, J.L. Removal of mercury(II) from contaminated water by gold-functionalised Fe3O4 magnetic nanoparticles. Environ. Technol. 2020, 41, 959–970. [Google Scholar] [CrossRef]
- Sadeghi, M.H.; Tofighy, M.A.; Mohammadi, T. One-Dimensional graphene for efficient aqueous heavy metal adsorption: Rapid removal of arsenic and mercury ions by graphene oxide nanoribbons (GONRs). Chemosphere 2020, 253, 126647. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Deliyanni, E.A. Mercury(II) removal with modified magnetic chitosan adsorbents. Molecules 2013, 18, 6193–6214. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Siafaka, P.I.; Lambropoulou, D.A.; Lazaridis, N.K.; Bikiaris, D.N. Poly(itaconic acid)-grafted chitosan adsorbents with different cross-linking for Pb(II) and Cd(II) uptake. Langmuir 2014, 30, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Kostoglou, M.; Lazaridis, N.K. Copper and chromium(VI) removal by chitosan derivatives-Equilibrium and kinetic studies. Chem. Eng. J. 2009, 152, 440–448. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Siafaka, P.I.; Pavlidou, E.G.; Chrissafis, K.J.; Bikiaris, D.N. Synthesis and adsorption application of succinyl-grafted chitosan for the simultaneous removal of zinc and cationic dye from binary hazardous mixtures. Chem. Eng. J. 2015, 259, 438–448. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Mone, M.; Koliakou, I.G.; Kyzas, G.Z.; Bikiaris, D.N. Synthesis and physicochemical properties of a new biocompatible chitosan grafted with 5-hydroxymethylfurfural. J. Mol. Liq. 2016, 222, 268–271. [Google Scholar] [CrossRef]
- Van Putten, R.J.; Van Der Waal, J.C.; De Jong, E.; Rasrendra, C.B.; Heeres, H.J.; De Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-Second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.S. Second-Order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Over the adsorption in solution. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Samuels, R.J. Solid state characterization of the structure of chitosan films. J. Polym. Sci. Polym. Phys. 1981, 19, 1081–1105. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Y.; Ma, G.; Shi, S.; Yang, D.; Lu, F.; Nie, J. A water-soluble photocrosslinkable chitosan derivative prepared by Michael-addition reaction as a precursor for injectable hydrogel. Carbohydr. Polym. 2010, 79, 507–512. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Rangel-Mendez, J.R.; Monroy-Zepeda, R.; Leyva-Ramos, E.; Diaz-Flores, P.E.; Shirai, K. Chitosan selectivity for removing cadmium (II), copper (II), and lead (II) from aqueous phase: pH and organic matter effect. J. Hazard. Mater. 2009, 162, 503–511. [Google Scholar] [CrossRef]
- Jin, L.; Bai, R. Mechanisms of lead adsorption on chitosan/PVA hydrogel beads. Langmuir 2002, 18, 9765–9770. [Google Scholar] [CrossRef]
- Kumar, M.; Tripathi, B.P.; Shahi, V.K. Crosslinked chitosan/polyvinyl alcohol blend beads for removal and recovery of Cd(II) from wastewater. J. Hazard. Mater. 2009, 172, 1041–1048. [Google Scholar] [CrossRef]
- He, J.; Lu, Y.; Luo, G. Ca(II) imprinted chitosan microspheres: An effective and green adsorbent for the removal of Cu(II), Cd(II) and Pb(II) from aqueous solutions. Chem. Eng. J. 2014, 244, 202–208. [Google Scholar] [CrossRef]
- Leyva-Ramos, R.; Rangel-Mendez, J.R.; Mendoza-Barron, J.; Fuentes-Rubio, L.; Guerrero-Coronado, R.M. Adsorption of cadmium(II) from aqueous solution onto activated carbon. Water Sci. Technol. 1997, 35, 205–211. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Tsai, W.T. A review of environmental hazards and adsorption recovery of cleaning solvent hydrochlorofluorocarbons (HCFCs). J. Loss Prev. Process Ind. 2002, 15, 147–157. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.; Cagnetta, G.; Wang, W.; Meng, P.; Liu, D.; Yu, G. Regeneration of chitosan-based adsorbents used in heavy metal adsorption: A review. Sep. Purif. Technol. 2019, 224, 373–387. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Lazaridis, N.K.; Kostoglou, M. Adsorption/desorption of a dye by a chitosan derivative: Experiments and phenomenological modeling. Chem. Eng. J. 2014, 248, 327–336. [Google Scholar] [CrossRef]
- Wu, Z.-C.; Wang, Z.-Z.; Liu, J.; Yin, J.-H.; Kuang, S.-P. Removal of Cu(II) ions from aqueous water by l-arginine modifying magnetic chitosan. Colloids Surf. A 2016, 499, 141–149. [Google Scholar] [CrossRef]
- Wan, M.W.; Kan, C.C.; Rogel, B.D.; Dalida, M.L.P. Adsorption of copper (II) and lead (II) ions from aqueous solution on chitosan-coated sand. Carbohydr. Polym. 2010, 80, 891–899. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, D.H.; Yoo, J.C.; Baek, K. Electrokinetic extraction of heavy metals from dredged marine sediment. Sep. Purif. Technol. 2011, 79, 164–169. [Google Scholar] [CrossRef]
- Yan, H.; Dai, J.; Yang, Z.; Yang, H.; Cheng, R. Enhanced and selective adsorption of copper(II) ions on surface carboxymethylated chitosan hydrogel beads. Chem. Eng. J. 2011, 174, 586–594. [Google Scholar] [CrossRef]













| Cu2+ | Cd2+ | |||||||
|---|---|---|---|---|---|---|---|---|
| Pseudo-1st Order | Pseudo-2nd Order | Pseudo-1st Order | PSEUDO-2ND ORDER | |||||
| K1 | R2 | K2 | R2 | K1 | R2 | K2 | R2 | |
| Adsorbent | (min−1) | (-) | (g mg−1 min−1) | (-) | (min−1) | (-) | (g mg−1 min−1) | (-) |
| CS0 | 0.01551 | 0.983 | 0.02789 | 0.952 | 0.02315 | 0.991 | 0.04075 | 0.918 |
| CS-HMF1ad | 0.04337 | 0.993 | 0.08099 | 0.931 | 0.04947 | 0.996 | 0.09361 | 0.951 |
| CS-HMF2ad | 0.07260 | 0.991 | 0.14141 | 0.930 | 0.06594 | 0.985 | 0.15812 | 0.893 |
| CS-HMF3ad | 0.08171 | 0.986 | 0.16296 | 0.919 | 0.06806 | 0.993 | 0.13228 | 0.936 |
| Cu2+ | Cd2+ | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Langmuir Equation | Freundlich Equation | Langmuir Equation | Freundlich Equation | ||||||||||
| T | Qm | KL | R2 | KF | n | R2 | Qm | KL | R2 | KF | n | R2 | |
| Adsorbent | (°C) | (mg/g) | (L/mg) | (-) | mg1−1/n L1/n g−1 | (-) | (-) | (mg/g) | (L/mg) | (-) | mg1−1/n L1/n g−1 | (-) | (-) |
| CS0 | 25 | 81 | 0.025 | 0.959 | 8.697 | 2.543 | 0.858 | 79 | 0.019 | 0.968 | 6.787 | 2.341 | 0.879 |
| 35 | 91 | 0.027 | 0.951 | 91 | 0.020 | 0.971 | |||||||
| 45 | 103 | 0.026 | 0.977 | 94 | 0.022 | 0.971 | |||||||
| CS-HMF1ad | 25 | 107 | 0.027 | 0.991 | 11.258 | 2.449 | 0.944 | 105 | 0.030 | 0.980 | 12.560 | 2.602 | 0.920 |
| 35 | 117 | 0.030 | 0.992 | 122 | 0.027 | 0.984 | |||||||
| 45 | 118 | 0.039 | 0.991 | 130 | 0.029 | 0.985 | |||||||
| CS-HMF2ad | 25 | 120 | 0.029 | 0.997 | 13.226 | 2.475 | 0.956 | 119 | 0.027 | 0.990 | 12.664 | 2.440 | 0.956 |
| 35 | 126 | 0.034 | 0.999 | 126 | 0.029 | 0.993 | |||||||
| 45 | 129 | 0.038 | 0.997 | 129 | 0.038 | 0.997 | |||||||
| CS-HMF3ad | 25 | 133 | 0.029 | 0.995 | 14.292 | 2.446 | 0.959 | 125 | 0.030 | 0.995 | 14.053 | 2.489 | 0.957 |
| 35 | 142 | 0.036 | 0.993 | 132 | 0.029 | 0.995 | |||||||
| 45 | 147 | 0.040 | 0.987 | 138 | 0.029 | 0.996 | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mone, M.; Lambropoulou, D.A.; Bikiaris, D.N.; Kyzas, G. Chitosan Grafted with Biobased 5-Hydroxymethyl-Furfural as Adsorbent for Copper and Cadmium Ions Removal. Polymers 2020, 12, 1173. https://doi.org/10.3390/polym12051173
Mone M, Lambropoulou DA, Bikiaris DN, Kyzas G. Chitosan Grafted with Biobased 5-Hydroxymethyl-Furfural as Adsorbent for Copper and Cadmium Ions Removal. Polymers. 2020; 12(5):1173. https://doi.org/10.3390/polym12051173
Chicago/Turabian StyleMone, Mariza, Dimitra A. Lambropoulou, Dimitrios N. Bikiaris, and George Kyzas. 2020. "Chitosan Grafted with Biobased 5-Hydroxymethyl-Furfural as Adsorbent for Copper and Cadmium Ions Removal" Polymers 12, no. 5: 1173. https://doi.org/10.3390/polym12051173
APA StyleMone, M., Lambropoulou, D. A., Bikiaris, D. N., & Kyzas, G. (2020). Chitosan Grafted with Biobased 5-Hydroxymethyl-Furfural as Adsorbent for Copper and Cadmium Ions Removal. Polymers, 12(5), 1173. https://doi.org/10.3390/polym12051173