Bioflocculants Produced by Bacterial Strains Isolated from Palm Oil Mill Effluent for Application in the Removal of Eriochrome Black T Dye from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. A Sampling of Palm Oil Mill Effluent (POME)
2.2. Isolation of Bioflocculant-Producing Bacteria
2.3. Morphological and Molecular Identification of Bioflocculant-Producing Bacteria
2.4. Flocculating Activity of Bioflocculant-Producing Bacteria in Kaolin Clay Suspension
2.5. Effect of Initial pH, Cations, and Dosage on the Flocculating Activity of Bioflocculant Bacteria towards Eriochrome Black T
2.6. Bioflocculant Purification and Characterization
3. Results and Discussion
3.1. Morphological and Molecular Characterization of Bioflocculant Bacteria
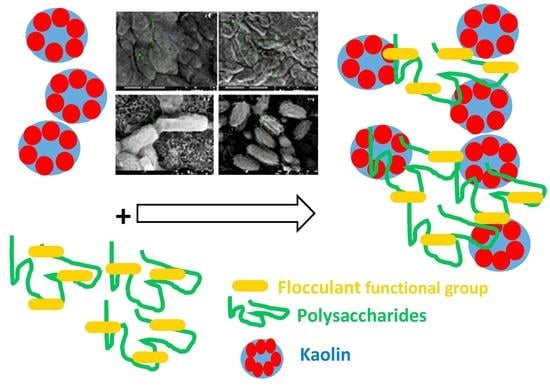
3.2. Flocculating Activity of Bioflocculant-Producing Bacteria in Kaolin Clay Suspension
3.3. Effect of Initial pH on the Flocculating Activity of Bacillus Nitratireducens towards Eriochrome Black T Synthetic Dye Wastewater
3.4. Effect of Cations on the Flocculating Activity of Bacillus Nitratireducens towards Eriochrome Black T Solution
3.5. Effect of Dosage and Cations on the Flocculating Activity of Bacillus Nitratireducens towards Eriochrome Black T Solution
3.6. Bioflocculant Characterization of Bacillus Nitratireducens
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malaysia, A.I. National Biomass Strategy 2020: New wealth creation for Malaysia’s palm oil industry. Agensi Inov. Kuala Lumpur Malays. 2011, 2, 2–34. [Google Scholar]
- Lau, Y.-Y.; Wong, Y.-S.; Teng, T.-T.; Morad, N.; Rafatullah, M.; Ong, S.-A. Coagulation-flocculation of azo dye Acid Orange 7 with green refined laterite soil. Chem. Eng. J. 2014, 246, 383–390. [Google Scholar] [CrossRef]
- Aljuboori, A.H.R.; Idris, A.; Al-joubory, H.H.R.; Uemura, Y.; Abubakar, B.I. Flocculation behavior and mechanism of bioflocculant produced by Aspergillus Flavus J. Environ. Manag. 2015, 150, 466–471. [Google Scholar] [CrossRef]
- Chaisorn, W.; Prasertsan, P.; Sompong, O.; Methacanon, P. Production and characterization of biopolymer as bioflocculant from thermotolerant Bacillus subtilis WD161 in palm oil mill effluent. Int. J. Hydrog. Energy 2016, 41, 21657–21664. [Google Scholar] [CrossRef]
- Oguz, E.; Keskinler, B. Removal of colour and COD from synthetic textile wastewaters using O3, PAC, H2O2 and HCO3−. J. Hazard Mater. 2008, 151, 753–760. [Google Scholar] [CrossRef]
- Lau, Y.-Y.; Wong, Y.-S.; Teng, T.-T.; Morad, N.; Rafatullah, M.; Ong, S.-A. Degradation of cationic and anionic dyes in coagulation–flocculation process using bi-functionalized silica hybrid with aluminum-ferric as auxiliary agent. RSC Adv. 2015, 5, 34206–34215. [Google Scholar] [CrossRef]
- Dulov, A.; Dulova, N.; Trapido, M. Combined physicochemical treatment of textile and mixed industrial wastewater. Ozone. Sci. Eng. 2011, 33, 285–293. [Google Scholar] [CrossRef]
- Ntsangani, N. Assessment of the Flocculating Efficiency of Bioflocculant Produced by Bacillus sp. Aemreg4 Isolated from Tyhume River, Eastern Cape, South Africa; University of Fort Hare: Alice, South Africa, 2016. [Google Scholar]
- Shahadat, M.; Teng, T.T.; Rafatullah, M.; Shaikh, Z.; Sreekrishnan, T.; Ali, S.W. Bacterial bioflocculants: A review of recent advances and perspectives. Chem. Eng. J. 2017, 328, 1139–1152. [Google Scholar] [CrossRef]
- Aljuboori, A.H.R.; Idris, A.; Abdullah, N.; Mohamad, R. Production and characterization of a bioflocculant produced by Aspergillus Flavus. Bioresour. Technol. 2013, 127, 489–493. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, L.; Chen, C.; Zhang, W.; Liu, M.; Han, Y.; Zhou, J. Production and characteristics of a bioflocculant by Klebsiella pneumoniae YZ-6 isolated from human saliva. Appl. Biochem. Biotechnol. 2014, 172, 1282–1292. [Google Scholar] [CrossRef]
- Okaiyeto, K.; Nwodo, U.U.; Mabinya, L.V.; Okoli, A.S.; Okoh, A.I. Characterization of a bioflocculant (MBF-UFH) produced by Bacillus sp. AEMREG7. Int. J. Mol. Sci. 2015, 16, 12986–13003. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Siddiqui, M.R.; Otero, M.; Alshareef, S.A.; Rafatullah, M. Removal of Rhodamine B from Water Using a Solvent Impregnated Polymeric Dowex 5WX8 Resin: Statistical Optimization and Batch Adsorption Studies. Polymers 2020, 12, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, J.N.; Dagang, W.R.Z.W. Role of cationization in bioflocculant efficiency: A review. Environ. Process. 2010, 6, 355–376. [Google Scholar] [CrossRef]
- Ben, R.F.; Mnif, W.; Siddeeg, S. Microbial flocculants as an alternative to synthetic polymers for wastewater treatment: A review. Symmetry 2018, 10, 556. [Google Scholar]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process. Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, H.; Zhou, J. Characterization of a bioflocculant MBF-5 by Klebsiella pneumoniae and its application in Acanthamoeba cysts removal. Bioresour. Technol. 2013, 137, 226–232. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Liu, X.; Zhou, J. Production and flocculating performance of sludge bioflocculant from biological sludge. Bioresour. Technol. 2013, 146, 51–56. [Google Scholar] [CrossRef]
- Alam, M.; Vandamme, D.; Chun, W.; Zhao, X.; Foubert, I.; Wang, Z.; Muylaert, K.; Yuan, Z. Bioflocculation as an innovative harvesting strategy for microalgae. Rev. Environ. Sci. Biotechnol. 2016, 15, 573–583. [Google Scholar] [CrossRef]
- Kurane, R.; Hatamochi, K.; Kakuno, T.; Kiyohara, M.; Hirano, M.; Taniguchi, Y. Production of a Bioflocculant by Rhodococcus erythropolis S-1 grown on alcohols. Biosci. Biotechnol. Biochem. 1994, 58, 428–429. [Google Scholar] [CrossRef]
- Bar-Or, Y.; Shilo, M. Characterization of macromolecular flocculants produced by Phormidium sp. Strain J-1 and by Anabaenopsis circularis PCC 6720. Appl. Environ. Microbiol. 1987, 53, 2226. [Google Scholar] [CrossRef] [Green Version]
- Salehizadeh, H.; Vossoughi, M.; Alemzadeh, I. Some investigations on bioflocculant producing bacteria. Biochem. Eng. J. 2000, 5, 39–44. [Google Scholar] [CrossRef]
- Yokoi, H.; Natsuda, O.; Hirose, J.; Hayashi, S.; Takasaki, Y. Characteristics of a biopolymer flocculant produced by Bacillus sp. PY-90. J. Ferment. Bioeng. 1995, 79, 378–380. [Google Scholar] [CrossRef]
- Yokoi, H.; Yoshida, T.; Mori, S.; Hirose, J.; Hayashi, S.; Takasaki, Y. Biopolymer flocculant produced by an Enterobacter sp. Biotechnol. Lett. 1997, 19, 569–573. [Google Scholar] [CrossRef]
- Shimofuruya, H.; Koide, A.; Shirota, K.; Tsuji, T.; Nakamura, M.; Suzuki, J. The production of flocculating substance (s) by Streptomyces griseus. Biosci. Biotechnol. Biochem. 1996, 60, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Shukla, S.K.; Singh, H.R.; Pradhan, K.K.; Jha, S.K. Production and purification of bioflocculants from newly isolated bacterial species: A comparative decolourization study of cationic and anionic textile dyes. Environ. Technol. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, S.; Thavamani, P.; Megharaj, M.; Venkateswarlu, K.; Lee, Y.B.; Naidu, R. Potential of Melaleuca diosmifolia as a novel, non-conventional and low-cost coagulating adsorbent for removing both cationic and anionic dyes. J. Ind. Eng. Chem. 2016, 37, 198–207. [Google Scholar] [CrossRef]
- Yokoi, H.; Arima, T.; Hirose, J.; Hayashi, S.; Takasaki, Y. Flocculation properties of poly (γ-glutamic acid) produced by Bacillus subtilis. J. Ferment. Bioeng. 1996, 82, 84–87. [Google Scholar] [CrossRef]
- Gong, W.-X.; Wang, S.-G.; Sun, X.-F.; Liu, X.-W.; Yue, Q.-Y.; Gao, B.-Y. Bioflocculant production by culture of Serratia ficaria and its application in wastewater treatment. Bioresour. Technol. 2008, 99, 4668–4674. [Google Scholar] [CrossRef]
- Deng, S.B.; Bai, R.B.; Hu, X.M.; Luo, Q. Characteristics of a bioflocculant produced by Bacillus mucilaginosus and its use in starch wastewater treatment. Appl. Microbiol. Biotechnol. 2003, 60, 588–593. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhang, Q.; Sheng, Y.; Li, C.; Wang, H. Screening and flocculating properties of bioflocculant-producing microorganisms. J. Uni. Sci. Technol. Beijing Min. Met. Mater. 2006, 13, 289–292. [Google Scholar] [CrossRef]
- Liu, W.; Wang, K.; Li, B.; Yuan, H.; Yang, J. Production and characterization of an intracellular bioflocculant by Chryseobacterium daeguense W6 cultured in low nutrition medium. Bioresour. Technol. 2010, 101, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy; Western Washington University: Bellingham, WA, USA, 2008. [Google Scholar]






| Physicochemical Property | Raw POME |
|---|---|
| Temperature (°C) | 40 ± 1 |
| pH | 8 ± 0.16 |
| Conductivity (μs/cm) | 130 ± 0.46 |
| Nitrogen (mg/L) | 9 ± 0.37 |
| Phosphate (PO4−3 mg/L) | 20.3 ± 0.25 |
| Total suspended solids (mg/L) | 188 ± 1 |
| Dissolved oxygen demand (mg/L) | 3.1 ± 0.15 |
| Chemical oxygen demand (mg/L) | 46,500 ± 3.55 |
| Biochemical oxygen demand (mg/L) | 21,000 ± 3.21 |
| Frequency (cm−1) | Functional Group |
|---|---|
| 1105.21–975.98 | C–O bonds |
| 1450.47–1398.39 | CH3 bending |
| 1589.34 | N–H bending |
| 1647.21 | C=O stretching, amide |
| 3273.20 | Carboxylic acid, O–H stretching |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, S.Z.; Yong, Y.-C.; Ali Khan, M.; Siddiqui, M.R.; Hakami, A.A.H.; Alshareef, S.A.; Otero, M.; Rafatullah, M. Bioflocculants Produced by Bacterial Strains Isolated from Palm Oil Mill Effluent for Application in the Removal of Eriochrome Black T Dye from Water. Polymers 2020, 12, 1545. https://doi.org/10.3390/polym12071545
Abbas SZ, Yong Y-C, Ali Khan M, Siddiqui MR, Hakami AAH, Alshareef SA, Otero M, Rafatullah M. Bioflocculants Produced by Bacterial Strains Isolated from Palm Oil Mill Effluent for Application in the Removal of Eriochrome Black T Dye from Water. Polymers. 2020; 12(7):1545. https://doi.org/10.3390/polym12071545
Chicago/Turabian StyleAbbas, Syed Zaghum, Yang-Chun Yong, Moonis Ali Khan, Masoom Raza Siddiqui, Afnan Ali Hussain Hakami, Shareefa Ahmed Alshareef, Marta Otero, and Mohd Rafatullah. 2020. "Bioflocculants Produced by Bacterial Strains Isolated from Palm Oil Mill Effluent for Application in the Removal of Eriochrome Black T Dye from Water" Polymers 12, no. 7: 1545. https://doi.org/10.3390/polym12071545
APA StyleAbbas, S. Z., Yong, Y. -C., Ali Khan, M., Siddiqui, M. R., Hakami, A. A. H., Alshareef, S. A., Otero, M., & Rafatullah, M. (2020). Bioflocculants Produced by Bacterial Strains Isolated from Palm Oil Mill Effluent for Application in the Removal of Eriochrome Black T Dye from Water. Polymers, 12(7), 1545. https://doi.org/10.3390/polym12071545