Diffusion and Controlled Release in Physically Crosslinked Poly (Vinyl Alcohol)/Iota-Carrageenan Hydrogel Blends
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Hydrogels Obtaining
2.3. Hydrogels Characterization
2.3.1. Solids Content and Gel Content Determination
2.3.2. Swelling Behavior
2.3.3. Controlled Release and Adsorption
2.3.4. Morpho-Structural Characterization
3. Results and Discussion
3.1. Hydrogels Swelling
3.2. Controlled Release from the Hydrogels
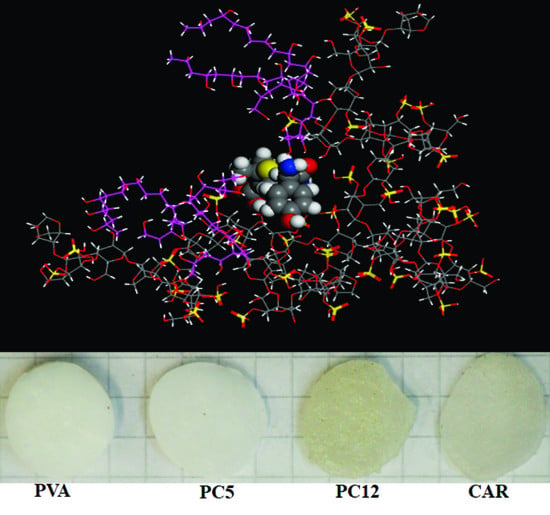
3.3. Gels Structure and Morphology
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moulay, S. Review: Poly(vinyl alcohol) Functionalizations and Applications. Polym. Plast Technol. 2015, 54, 1289–1319. [Google Scholar] [CrossRef]
- Masri, C.; Chagnon, G.; Favier, D. Influence of processing parameters on the macroscopic mechanical behavior of PVA hydrogels. Mater. Sci. Eng. C Mater. 2017, 75, 769–776. [Google Scholar] [CrossRef] [Green Version]
- Mori, Y.; Tokura, H.; Yoshikawa, M. Properties of hydrogels synthesized by freezing and thawing aqueous polyvinyl alcohol solutions and their applications. J. Mater. Sci. 1997, 32, 491–496. [Google Scholar] [CrossRef]
- Hu, W.K.; Wang, Z.J.; Xiao, Y.; Zhang, S.M.; Wang, J.L. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. UK 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Timofejeva, A.; D’Este, M.; Loca, D. Calcium phosphate/polyvinyl alcohol composite hydrogels: A review on the freeze–thaw synthesis approach and applications in regenerative medicine. Eur. Polym. J. 2017, 95, 547–565. [Google Scholar] [CrossRef]
- Peng, H.F.; Wang, S.P.; Xu, H.Y.; Hao, X.W. Preparation, properties and formation mechanism of cellulose/polyvinyl alcohol bio-composite hydrogel membranes. New J. Chem. 2017, 41, 6564–6573. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Ko, H.U.; Kim, H.C.; Kim, J.W.; Li, Y.G.; Kim, J. Transparent and semi-interpenetrating network P(vinyl alcohol)- P(Acrylic acid) hydrogels: pH responsive and electroactive application. Int. J. Smart Nano Mater. 2017, 8, 80–94. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.M.N.; Moreira, A.P.D.; Carvalho, C.W.P.; Luchese, R.; Ribeiro, E.; McGuinness, G.B.; Mendes, M.F.; Oliveira, R.N. Physically cross-linked gels of PVA with natural polymers as matrices for manuka honey release in wound-care applications. Materials 2019, 12, 559. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Park, S.J.; Kim, S.I. Swelling behavior of interpenetrating polymer network hydrogels composed of poly(vinyl alcohol) and chitosan. React. Funct. Polym. 2003, 55, 53–59. [Google Scholar] [CrossRef]
- Baron, R.I.; Culica, M.E.; Biliuta, G.; Bercea, M.; Gherman, S.; Zavastin, D.; Ochiuz, L.; Avadanei, M.; Coseri, S. Physical Hydrogels of Oxidized Polysaccharides and Poly(Vinyl Alcohol) for Wound Dressing Applications. Materials 2019, 12, 1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varshney, L. Role of natural polysaccharides in radiation formation of PVA-hydrogel wound dressing. Nucl. Instrum. Meth. B 2007, 255, 343–349. [Google Scholar] [CrossRef]
- Yang, K.R.; Han, Q.; Chen, B.P.; Zheng, Y.H.; Zhang, K.S.; Li, Q.; Wang, J.C. Antimicrobial hydrogels: Promising materials for medical application. Int. J. Nanomed. 2018, 13, 2217–2263. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.Y.; Wang, M.; Jia, F.; Ren, X.Y.; Gao, G.H. Thermo-responsive shape memory sensors based on tough, remolding and anti-freezing hydrogels. J. Mater. Chem. C 2020, 8, 2326–2335. [Google Scholar] [CrossRef]
- Krishna, K.A.; Vishalakshi, B. Gellan gum-based novel composite hydrogel: Evaluation as adsorbent for cationic dyes. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Chowdhury, M.N.K.; Ismail, A.F.; Beg, M.D.H.; Hegde, G.; Gohari, R.J. Polyvinyl alcohol/polysaccharide hydrogel graft materials for arsenic and heavy metal removal. New J. Chem. 2015, 39, 5823–5832. [Google Scholar] [CrossRef] [Green Version]
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Tran, V.V.; Park, D.; Lee, Y.C. Hydrogel applications for adsorption of contaminants in water and wastewater treatment. Environ. Sci. Pollut. R 2018, 25, 24569–24599. [Google Scholar] [CrossRef]
- Moreira, R.; Chenlo, F.; Torres, M.D. Gelling characteristics and rheology of kappa/iota-hybrid carrageenans extracted from Mastocarpus stellatus dried at different temperatures. J. Appl. Phycol. 2016, 28, 3635–3644. [Google Scholar] [CrossRef]
- Prasad, K.; Kaneko, Y.; Kadokawa, J. Novel Gelling Systems of kappa-, iota- and lambda-Carrageenans and their Composite Gels with Cellulose Using Ionic Liquid. Macromol. Biosci. 2009, 9, 376–382. [Google Scholar] [CrossRef]
- Shahbazi, M.; Rajabzadeh, G.; Rafe, A.; Ettelaie, R.; Ahmadi, S.J. The physico-mechanical and structural characteristics of blend film of poly (vinyl alcohol) with biodegradable polymers as affected by disorder-to-order conformational transition. Food Hydrocolloid 2016, 60, 393–404. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Lu, T.; Yuasa, S.; Yamaura, K. Structure and properties of poly(vinyl alcohol)/kappa-carrageenan blends. Polym. Int. 2001, 50, 1103–1108. [Google Scholar] [CrossRef]
- Kantoglu, O.; Caykara, T.; Guven, O. Preparation and characterization of polysaccaride interpolymer complexes: I-PVA/iota-carrageenan. J. Appl. Polym. Sci. 2013, 127, 500–507. [Google Scholar] [CrossRef]
- Wu, N.H.; Bao, B.R.; Yoshii, F.; Makuuchi, K. Irradiation of crosslinked, poly(vinyl alcohol) blended hydrogel for wound dressing. J. Radioanal Nucl. Ch. 2001, 250, 391–395. [Google Scholar] [CrossRef]
- Gorin, K.V.; Badranova, G.U.; Gotovtsev, P.M.; Shatalova, A.Y.; Grigoriev, T.E.; Krasheninnikov, S.V.; Tihomirov, S.A.; Kondratev, O.A.; Vishnevskaya, M.V.; Vasilov, R.G. The CRG-PVA hydrogels study of properties with various nanoparticles and their application for cultivation of phototrophic microorganisms. In Proceedings of the 2nd International Conference on New Material and Chemical Industry, Sanya, China, 18–20 November 2017. [Google Scholar]
- Chopra, P.; Nayak, D.; Nanda, A.; Ashe, S.; Rauta, P.R.; Nayak, B. Fabrication of poly(vinyl alcohol)-Carrageenan scaffolds for cryopreservation: Effect of composition on cell viability. Carbohydr. Polym. 2016, 147, 509–516. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Dehariya, P.; Saggu, S.P.S. Investigation of Moisture Sorption, Permeability, Cytotoxicity and Drug Release Behavior of Carrageenan/Poly Vinyl Alcohol Films. J. Macromol. Sci. A 2015, 52, 243–251. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Daheriya, P.; Ahuja, S.; Gupta, K. Water absorption and antimicrobial behavior of physically cross linked poly (vinyl alcohol)/carrageenan films loaded with minocycline. Des. Monomers Polym. 2016, 19, 630–642. [Google Scholar] [CrossRef] [Green Version]
- Croitoru, C.; Pop, M.A.; Bedo, T.; Cosnita, M.; Roata, I.C.; Hulka, I. Physically Crosslinked Poly (Vinyl Alcohol)/Kappa-Carrageenan Hydrogels: Structure and Applications. Polymers 2020, 12, 560. [Google Scholar] [CrossRef] [Green Version]
- Badranova, G.U.; Gotovtsev, P.M.; Zubavichus, Y.V.; Staroselskiy, I.A.; Vasiliev, A.L.; Trunkin, I.N.; Fedorov, M.V. Biopolymer-based hydrogels for encapsulation of photocatalytic TiO2 nanoparticles prepared by the freezing/thawing method. J. Mol. Liq. 2016, 223, 16–20. [Google Scholar] [CrossRef]
- Wang, C.R.; Zhang, M.R.; Gao, Z.F.; Ren, X. Comparison of degradation kinetics of free and immobilized serratia sp for phenol removal from coking wastewater. Fresen. Environ. Bull. 2015, 24, 1629–1635. [Google Scholar]
- Khan, S.; Ranjha, N.M. Effect of degree of cross-linking on swelling and on drug release of low viscous chitosan/poly(vinyl alcohol) hydrogels. Polym. Bull. 2014, 71, 2133–2158. [Google Scholar] [CrossRef]
- Krusic, M.K.; Ilic, M.; Filipovic, J. Swelling behaviour and paracetamol release from poly(N-isopropylacrylamide-itaconic acid) hydrogels. Polym. Bull. 2009, 63, 197–211. [Google Scholar] [CrossRef]
- Liu, Y.; Vrana, N.E.; Cahill, P.A.; McGuinness, G.B. Physically Crosslinked Composite Hydrogels of PVA With Natural Macromolecules: Structure, Mechanical Properties, and Endothelial Cell Compatibility. J. Biomed. Mater. Res. B 2009, 90b, 492–502. [Google Scholar] [CrossRef]
- Sun, M.; Li, D.P.; Wang, X.; He, L.; Lv, X.D.; Xu, Y.; Tang, R.P. Intestine-penetrating, pH-sensitive and double-layered nanoparticles for oral delivery of doxorubicin with reduced toxicity. J. Mater. Chem. B 2019, 7, 3692–3703. [Google Scholar] [CrossRef]
- Emara, K.M.; Askal, H.F.; Saleh, G.A. Spectrophotometric Determination of Tetracycline and Oxytetracycline in Pharmaceutical Preparations. Talanta 1991, 38, 1219–1221. [Google Scholar] [CrossRef]
- ElShafie, F.S.; GadKariem, E.A.; AlRashood, K.A.; AlKhamees, H.A.; ElObeid, H.A. Colorimetric method for the determination of ampicillin and amoxicillin. Anal. Lett. 1996, 29, 381–393. [Google Scholar] [CrossRef]
- Ismail, A.F.H.; Mohamed, F.; Rosli, L.M.M.; Shafri, M.A.M.; Haris, M.S.; Adina, A.B. Spectrophotometric Determination of Gentamicin Loaded PLGA Microparticles and Method Validation via Ninhydrin-Gentamicin Complex as a Rapid Quantification Approach. J. Appl. Pharm. Sci. 2016, 6, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Bono, A.; Anisuzzaman, S.M.; Ding, O.W. Effect of process conditions on the gel viscosity and gel strength of semi-refined carrageenan (SRC) produced from seaweed (Kappaphycus alvarezii). J. King Saud Univ. Eng. Sci. 2014, 26, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Pamies, R.; Cifre, J.G.H.; Martinez, M.D.L.; de la Torre, J.G. Determination of intrinsic viscosities of macromolecules and nanoparticles. Comparison of single-point and dilution procedures. Colloid Polym. Sci. 2008, 286, 1223–1231. [Google Scholar] [CrossRef]
- Bercea, M.; Morariu, S.; Rusu, D. In situ gelation of aqueous solutions of entangled poly(vinyl alcohol). Soft Matter 2013, 9, 1244–1253. [Google Scholar] [CrossRef]
- Jain, E.; Kumar, A. Designing Supermacroporous Cryogels Based on Polyacrylonitrile and a Polyacrylamide-Chitosan Semi-interpenetrating Network. J. Biomat. Sci. Polym. E 2009, 20, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.I. Effect of Non-Uniform Initial-Drug Concentration Distribution on the Kinetics of Drug Release from Glassy Hydrogel Matrices. Polymer 1984, 25, 973–978. [Google Scholar] [CrossRef]
- Varshosaz, J.; Hajian, M. Characterization of drug release and diffusion mechanism through hydroxyethylmethacrylate/methacrylic acid pH-sensitive hydrogel. Drug Deliv. 2004, 11, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Yakar, A.; Guven, O. Determination of average molecular weight between cross-links ((M)over-bar(c)) from swelling behaviours of diprotic acid-containing hydrogels. Polymer 1999, 40, 2969–2974. [Google Scholar] [CrossRef]
- Siepmann, J.; Podual, K.; Sriwongjanya, M.; Peppas, N.A.; Bodmeier, R. A new model describing the swelling and drug release kinetics from hydroxypropyl methylcellulose tablets. J. Pharm. Sci. US 1999, 88, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Berens, A.R.; Hopfenberg, H.B. Diffusion and Relaxation in Glassy Polymer Powders. 2. Separation of Diffusion and Relaxation Parameters. Polymer 1978, 19, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Mircioiu, C.; Voicu, V.; Anuta, V.; Tudose, A.; Celia, C.; Paolino, D.; Fresta, M.; Sandulovici, R.; Mircioiu, I. Mathematical Modeling of Release Kinetics from Supramolecular Drug Delivery Systems. Pharmaceutics 2019, 11, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, L.; Ahmad, M.; Usman, M.; Yousuf, M. Controlled release of highly water-soluble antidepressant from hybrid copolymer poly vinyl alcohol hydrogels. Polym. Bull. 2014, 71, 31–46. [Google Scholar] [CrossRef]
- Paradee, N.; Sirivat, A.; Niamlang, S.; Prissanaroon-Ouajai, W. Effects of crosslinking ratio, model drugs, and electric field strength on electrically controlled release for alginate-based hydrogel. J. Mater. Sci. Mater. M 2012, 23, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Subuddhi, U. Guar gum-poly(N-isopropylacrylamide) smart hydrogels for sustained delivery of 5-fluorouracil. Polym. Bull. 2019, 76, 2945–2963. [Google Scholar] [CrossRef]
- Altinisik, A.; Yurdakoc, K. Chitosan/poly(vinyl alcohol) hydrogels for amoxicillin release. Polym. Bull. 2014, 71, 759–774. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y. Measurement and correlation of solubility of Tetracycline hydrochloride in six organic solvents. J. Chem. Thermodyn. 2013, 57, 9–13. [Google Scholar] [CrossRef]
- Homem, V.; Alves, A.; Santos, L. Amoxicillin degradation at ppb levels by Fenton’s oxidation using design of experiments. Sci. Total Environ. 2010, 408, 6272–6280. [Google Scholar] [CrossRef]
- Rosenkrantz, B.E.; Greco, J.R.; Hoogerheide, J.G.; Oden, E.M. Gentamicin Sulfate. Anal. Profiles Drug Subst. Excipients 1981, 9, 295–340. [Google Scholar] [CrossRef]
- Jayaraju, J.; Keshavayya, J.; Rai, S.K.; Basavaraju, K.C. Miscibility studies on chitosan/poly(vinyl alcohol) blends. J. Macromol. Sci. A 2008, 45, 271–275. [Google Scholar] [CrossRef]
- Watase, M.; Nishinari, K. Large Deformation of Hydrogels of Polyvinyl-Alcohol), Agarose and Kappa-Carrageenan. Makromol. Chem. 1985, 186, 1081–1086. [Google Scholar] [CrossRef]
- Lewandowska, K. Viscometric Studies in Dilute Solution Mixtures of Chitosan and Microcrystalline Chitosan with Poly(vinyl alcohol). J. Solution Chem. 2013, 42, 1654–1662. [Google Scholar] [CrossRef] [Green Version]
- Hassan, C.M.; Peppas, N.A. Structure and morphology of freeze/thawed PVA hydrogels. Macromolecules 2000, 33, 2472–2479. [Google Scholar] [CrossRef]
- Mangione, M.R.; Giacomazza, D.; Bulone, D.; Martorana, V.; San Biagio, P.L. Thermoreversible gelation of kappa-Carrageenan: Relation between conformational transition and aggregation. Biophys. Chem. 2003, 104, 95–105. [Google Scholar] [CrossRef]
- Chronakis, I.S.; Doublier, J.L.; Piculell, L. Viscoelastic properties for kappa- and iota-carrageenan in aqueous NaI from the liquid-like to the solid-like behaviour. Int. J. Biol. Macromol. 2000, 28, 1–14. [Google Scholar] [CrossRef]
- Figueroa-Pizano, M.D.; Velaz, I.; Martinez-Barbosa, M.E. A Freeze-Thawing Method to Prepare Chitosan-Poly(vinyl alcohol) Hydrogels Without Crosslinking Agents and Diflunisal Release Studies. Jove J. Vis. Exp. 2020. [Google Scholar] [CrossRef]
- Kenawy, E.; Kamoun, E.A.; Eldin, M.S.M.; El-Meligy, M.A. Physically crosslinked poly(vinyl alcohol)-hydroxyethyl starch blend hydrogel membranes: Synthesis and characterization for biomedical applications. Arab. J. Chem. 2014, 7, 372–380. [Google Scholar] [CrossRef] [Green Version]
- Atlan, M.; Simon-Yarza, T.; Ino, J.M.; Hunsinger, V.; Corte, L.; Ou, P.; Aid-Launais, R.; Chaouat, M.; Letourneur, D. Design, characterization and in vivo performance of synthetic 2 mm-diameter vessel grafts made of PVA-gelatin blends. Sci. Rep. UK 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.F.; Du, Y.M.; Li, Y.; Wang, X.Y.; Hu, X.W. A thermosensitive chitosan/poly(vinyl alcohol) hydrogel containing hydroxyapatite for protein delivery. J. Biomed. Mater. Res. A 2009, 91a, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, R.; Auriemma, F.; De Rosa, C.; Laupretre, F. X-ray diffraction analysis of poly(vinyl alcohol) hydrogels, obtained by freezing and thawing techniques. Macromolecules 2004, 37, 1921–1927. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Ko, H.U.; Zhai, L.D.; Li, Y.G.; Kim, J. Preparation and characterization of hydrogels from polyvinyl alcohol and cellulose and their electroactive behavior. Soft Mater. 2017, 15, 64–72. [Google Scholar] [CrossRef]
- Tretinnikov, O.N.; Zagorskaya, S.A. Determination of the degree of crystallinity of poly(Vinyl alcohol) byFTIR spectroscopy. J. Appl. Spectrosc. 2012, 79, 521–526. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S.; Gordeeva, A.M.; Faizullin, D.A.; Gusev, Y.A.; Zuev, Y.F.; Makshakova, O.N. Molecular structure and properties of κ-carrageenan-gelatin gels. Carbohydr. Polym. 2018, 197, 66–74. [Google Scholar] [CrossRef] [PubMed]











| Sample Code | Polymeric Components Amount | wCAR | mdrug (g) | δ (mm) | SC (%) | GC (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| PVA | CAR | ||||||||
| PVA Solution Volume (mL) | PVA Amount (g) | CAR Solution Volume (mL) | CAR Amount (g) | ||||||
| PVA | 10 | 1.000 | – | – | 0 | 0.250 | 3.87 | 9.81 | 87.21 |
| PC5 | 8 | 0.800 | 2 | 0.040 | 0.047 | 0.210 | 3.81 | 8.22 | 82.78 |
| PC12 | 6 | 0.600 | 4 | 0.080 | 0.117 | 0.170 | 3.74 | 5.63 | 83.31 |
| PC12K | 6 | 0.600 | 4 | 0.080 | 0.117 | 0.170 ** | 3.79 | 5.43 | 79.81 |
| CAR | – | – | 10 | 0.200 | 1 | – | 3.62 | 2.04 | 0.83 |
| Sample Code | ϕs⋅102 | χ | ν⋅104 | Mc | ζ (nm) |
|---|---|---|---|---|---|
| PVA | 7.210 | 0.525 | 23.700 | 445.13 | 13.970 |
| PC5 | 4.320 | 0.528 | 8.091 | 1295.15 | 28.270 |
| PC12 | 2.212 | 0.530 | 1.998 | 5204.83 | 70.810 |
| PC12K | 2.013 | 0.544 | 1.274 | 6378.27 | 80.312 |
| PC12-A | 2.032 | 0.505 | 1.650 | 6729.57 | 82.980 |
| PC12-T | 2.203 | 0.508 | 1.980 | 5630.57 | 73.770 |
| PC12-G | 2.069 | 0.507 | 1.730 | 6439.89 | 80.601 |
| CAR | 1.140 | 0.504 | 0.846 | 11,977.17 | – |
| Material | Parameter | Swelling Medium | |||||
|---|---|---|---|---|---|---|---|
| Distilled Water | pH = 3 | pH = 4 | pH = 5 | pH = 6.5 | pH = 7.3 | ||
| PVA | kD (min−n) | 0.42 (0.990) | 0.54 (0.996) | 0.50 (0.998) | 0.47 (0.993) | 0.45 (0.994) | 0.46 (0.992) |
| n | 0.92 (0.990) | 0.97 (0.996) | 0.94 (0.998) | 0.95 (0.993) | 0.99 (0.994) | 0.94 (0.992) | |
| SDdiff (%) | 23.09 (0.988) | 21.90 (0.981) | 22.49 (0.990) | 22.67 (0.987) | 22.78 (0.991) | 22.97 (0.996) | |
| SDrel,∞(%) | 122.53 (0.988) | 126.45 (0.981) | 125.57 (0.990) | 124.73 (0.987) | 123.98 (0.991) | 122.13 (0.996) | |
| κ× 102 (min−1) | 1.66 (0.988) | 1.79 (0.981) | 1.72 (0.990) | 1.71 (0.987) | 1.68 (0.991) | 1.70 (0.996) | |
| to(min) | 88.19 (0.988) | 79.12 (0.981) | 79.83 (0.990) | 83.11 (0.987) | 85.01 (0.991) | 88.04 (0.996) | |
| D× 105 (cm2/s) | 8.64 | 10.13 | 10.04 | 9.58 | 8.69 | 8.72 | |
| PC5 | kD(min−n) | 0.55 (0.961) | 0.68 (0.994) | 0.63 (0.989) | 0.59 (0.995) | 0.57 (0.998) | 0.55 (0.990) |
| n | 0.87 (0.961) | 0.94 (0.994) | 0.92 (0.989) | 0.90 (0.995) | 0.89 (0.998) | 0.86 (0.990) | |
| SDdiff (%) | 46.21 (0.955) | 40.34 (0.994) | 41.77 (0.997) | 43.11 (0.991) | 43.52 (0.994) | 46.73 (0.992) | |
| SDrel,∞(%) | 137.60 (0.955) | 149.56 (0.993) | 140.34 (0.997) | 139.83 (0.991) | 138.84 (0.994) | 137.97 (0.992) | |
| κ× 103 (min−1) | 5.90 (0.955) | 8.31 (0.993) | 8.04 (0.997) | 7.56 (0.991) | 7.00 (0.994) | 6.43 (0.992) | |
| to(min) | 60.91 (0.955) | 39.90 (0.993) | 45.58 (0.997) | 51.78 (0.991) | 55.00 (0.994) | 58.72 (0.992) | |
| D× 105 (cm2/s) | 8.90 | 18.34 | 16.98 | 12.65 | 10.19 | 9.04 | |
| PC12 | kD(min−n) | 1.26 (0.966) | 1.42 (0.992) | 1.38 (0.995) | 1.35 (0.988) | 1.30 (0.991) | 1.29 (0.997) |
| n | 0.80 (0.966) | 0.89 (0.992) | 0.87 (0.995) | 0.85 (0.988) | 0.83 (0.991) | 0.83 (0.997) | |
| SDdiff (%) | 27.88 (0.949) | 20.11 (0.988) | 21.98 (0.994) | 22.65 (0.989) | 24.77 (0.990) | 27.10 (0.992) | |
| SDrel,∞(%) | 153.08 (0.949) | 160.56 (0.988) | 158.77 (0.994) | 156.75 (0.989) | 155.04 (0.990) | 154.07 (0.992) | |
| κ× 103 (min−1) | 5.60 (0.949) | 7.02 (0.988) | 6.72 (0.994) | 6.28 (0.989) | 5.91 (0.990) | 5.67 (0.992) | |
| to(min) | 29.37 (0.949) | 15.34 (0.988) | 18.62 (0.994) | 22.86 (0.989) | 24.11 (0.990) | 28.75 (0.992) | |
| D× 104 (cm2/s) | 1.04 | 5.87 | 4.33 | 2.85 | 2.07 | 1.13 | |
| PC12K | kD(min−n) | 1.34 (0.998) | 1.53 (0.991) | 1.50 (0.990) | 1.42 (0.989) | 1.38 (0.992) | 1.35 (0.997) |
| n | 0.93 (0.998) | 0.96 (0.991) | 0.95 (0.990) | 0.94 (0.989) | 0.94 (0.992) | 0.93 (0.997) | |
| SDdiff (%) | 22.65 (0.994) | 19.83 (0.998) | 19.97 (0.995) | 20.12 (0.0990) | 20.21 (0.996) | 22.03 (0.992) | |
| SDrel,∞(%) | 172.86 (0.994) | 181.49 (0.998) | 176.42 (0.995) | 173.16 (0.990) | 173.04 (0.996) | 173.21 (0.992) | |
| κ× 102 (min−1) | 2.07 (0.994) | 4.09 (0.998) | 3.92 (0.995) | 3.56 (0.990) | 3.28 (0.996) | 2.09 (0.992) | |
| to(min) | 14.78 (0.994) | 7.39 (0.998) | 8.12 (0.995) | 9.77 (0.990) | 12.27 (0.996) | 12.85 (0.992) | |
| D× 103 (cm2/s) | 4.07 | 4.88 | 4.74 | 4.51 | 4.16 | 4.10 | |
| CAR | kD(min−n) | 2.73 (0.991) | n.a. (gel not stable) | ||||
| n | 0.82 (0.991) | ||||||
| SDdiff (%) | 26.85 (0.831) | ||||||
| SDrel,∞(%) | 158.71 (0.831) | ||||||
| κ× 102 (min−1) | 2.20 (0.831) | ||||||
| to(min) | 11.15 (0.831) | ||||||
| D× 103 (cm2/s) | 3.86 | ||||||
| Material | Parameter | Swelling Medium | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH = 3 | pH = 6.5 | pH = 7.3 | ||||||||
| PVA | Antibiotic | A | T | G | A | T | G | A | T | G |
| k1× 102 (min−m) | 7.04(0.993) | 6.04(0.995) | 3.27(0.997) | 5.89(0.992) | 4.50(0.992) | 2.89(0.994) | 5.23(0.992) | 4.39(0.996) | 2.78(0.997) | |
| k2× 103(min−2n) | 7.98(0.993) | 7.26(0.995) | 2.82(0.997) | 7.84(0.992) | 6.22(0.992) | 2.34(0.994) | 7.36(0.992) | 6.12(0.996) | 1.10(0.997) | |
| n | 0.58(0.993) | 0.56(0.995) | 0.54(0.997) | 0.60(0.992) | 0.58(0.992) | 0.54(0.994) | 0.63(0.992) | 0.60(0.996) | 0.56(0.997) | |
| Ddiff× 106 (cm2/s) | 8.97(0.999) | 8.73(0.987) | 4.17(0.994) | 8.33(0.987) | 7.18(0.988) | 3.94(0.990) | 8.04(0.988) | 6.24(0.989) | 3.76(0.996) | |
| Drel× 106 (cm2/s) | 4.78(0.988) | 4.21(0.989) | 1.55(0.989) | 4.16(0.988) | 4.06(0.991) | 1.48(0.986) | 4.11(0.991) | 3.04(0.998) | 1.26(0.994) | |
| PC5 | k1× 102 (min−m) | 8.12(0.994) | 6.85(0.997) | 3.94(0.994) | 6.05(0.996) | 4.82(0.992) | 3.67(0.991) | 5.68(0.992) | 4.77(0.995) | 2.92(0.999) |
| k2× 103(min−2n) | 9.04(0.994) | 7.08(0.997) | 2.78(0.994) | 7.02(0.996) | 5.56(0.992) | 2.28(0.991) | 6.98(0.992) | 4.01(0.995) | 1.02(0.999) | |
| n | 0.55(0.994) | 0.54(0.997) | 0.54(0.994) | 0.56(0.996) | 0.55(0.992) | 0.52(0.991) | 0.58(0.992) | 0.56(0.995) | 0.54(0.999) | |
| Ddiff× 106 (cm2/s) | 9.31(0.992) | 8.89(0.991) | 4.28(0.992) | 8.87(0.990) | 7.24(0.991) | 4.08(0.993) | 8.12(0.991) | 6.79(0.989) | 3.83(0.998) | |
| Drel× 106 (cm2/s) | 4.82(0.991) | 4.12(0.992) | 1.31(0.990) | 4.06(0.994) | 3.51(0.997) | 1.24(0.991) | 4.02(0.994) | 2.97(0.987) | 1.13(0.993) | |
| PC12 | k1× 102 (min−m) | 8.44(0.998) | 6.90(0.992) | 3.98(0.996) | 6.87(0.998) | 5.34(0.994) | 3.78(0.998) | 5.91(0.994) | 4.82(0.991) | 2.98(0.988) |
| k2× 103(min−2n) | 9.26(0.998) | 6.98(0.992) | 2.42(0.996) | 8.03(0.998) | 4.84(0.994) | 2.08(0.998) | 6.71(0.994) | 3.96(0.991) | 0.97(0.998) | |
| n | 0.53(0.998) | 0.53(0.992) | 0.53(0.996) | 0.54(0.998) | 0.53(0.994) | 0.53(0.998) | 0.56(0.994) | 0.55(0.991) | 0.52(0.998) | |
| Ddiff× 106 (cm2/s) | 9.38(0.994) | 8.96(0.993) | 4.36(0.994) | 8.90(0.987) | 6.91(0.990) | 4.11(0.988) | 8.24(0.990) | 6.83(0.992) | 3.91(0.996) | |
| Drel× 106 (cm2/s) | 4.92(0.990) | 4.06(0.991) | 1.18(0.987) | 3.89(0.986) | 3.16(0.991) | 1.10(0.990) | 2.98(0.988) | 2.90(0.986) | 1.04(0.998) | |
| PC12K | k1× 101 (min−m) | – | 1.02(0.998) | – | – | 0.82(0.992) | – | – | 0.73(0.998) | – |
| k2× 102 (min−2n) | – | 3.28(0.998) | – | – | 2.81(0.992) | – | – | 2.46(0.998) | – | |
| n | – | 0.61(0.998) | – | – | 0.60(0.997) | – | – | 0.56(0.998) | – | |
| Ddiff× 105 (cm2/s) | – | 2.18(0.989) | – | – | 2.08(0.994) | – | – | 1.92(0.993) | – | |
| Drel× 105 (cm2/s) | – | 6.82(0.991) | – | – | 6.77(0.996) | – | – | 6.21(0.995) | – | |
| Blend Composition | (η) (dL/g) | ΚH | b (dL2/g2) | μ | R2 | |
|---|---|---|---|---|---|---|
| wPVA | wCAR | |||||
| 1 | 0 | 0.793 | 0.427 | 0.268 | – | 0.998 |
| 0.990 | 0.010 | 1.107 | 0.214 | 0.263 | −0.02 | 0.989 |
| 0.978 | 0.021 | 1.311 | 1.081 | 1.860 | 4.06 | 0.998 |
| 0.953 | 0.047 | 1.552 | 1.090 | 2.627 | 2.81 | 0.999 |
| 0.883 | 0.117 | 1.846 | 0.965 | 3.290 | 1.44 | 0.996 |
| 0.860 | 0.140 | 2.026 | 0.869 | 3.574 | 1.31 | 0.995 |
| 0.834 | 0.166 | 2.583 | 0.444 | 2.968 | 0.85 | 0.981 |
| 0.767 | 0.233 | 3.032 | 0.437 | 4.017 | 0.63 | 0.986 |
| 0 | 1 | 3.816 | 1.346 | 19.618 | – | 0.978 |
| Sample Code | Pr (%) | CrXRD (%) | D (nm) | CrIFTIR | EH (kcal) | |
|---|---|---|---|---|---|---|
| PVA | 8.23 | 11.03 | 7.94 | 0.24 | 6.12 | |
| PC5 | 6.04 | 9.94 | 7.68 | 0.21 | 6.22 | |
| PC12 | 5.14 | 7.27 | 7.57 | 0.15 | 3.96 | |
| CAR | 0.73 | 4.05 | – | – | 4.56 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croitoru, C.; Roata, I.C.; Pascu, A.; Stanciu, E.M. Diffusion and Controlled Release in Physically Crosslinked Poly (Vinyl Alcohol)/Iota-Carrageenan Hydrogel Blends. Polymers 2020, 12, 1544. https://doi.org/10.3390/polym12071544
Croitoru C, Roata IC, Pascu A, Stanciu EM. Diffusion and Controlled Release in Physically Crosslinked Poly (Vinyl Alcohol)/Iota-Carrageenan Hydrogel Blends. Polymers. 2020; 12(7):1544. https://doi.org/10.3390/polym12071544
Chicago/Turabian StyleCroitoru, Catalin, Ionut Claudiu Roata, Alexandru Pascu, and Elena Manuela Stanciu. 2020. "Diffusion and Controlled Release in Physically Crosslinked Poly (Vinyl Alcohol)/Iota-Carrageenan Hydrogel Blends" Polymers 12, no. 7: 1544. https://doi.org/10.3390/polym12071544
APA StyleCroitoru, C., Roata, I. C., Pascu, A., & Stanciu, E. M. (2020). Diffusion and Controlled Release in Physically Crosslinked Poly (Vinyl Alcohol)/Iota-Carrageenan Hydrogel Blends. Polymers, 12(7), 1544. https://doi.org/10.3390/polym12071544