Hybrid Electromagnetic Nanomaterials Based on Polydiphenylamine-2-carboxylic Acid
Abstract
:1. Introduction
2. Experimental
2.1. Materials
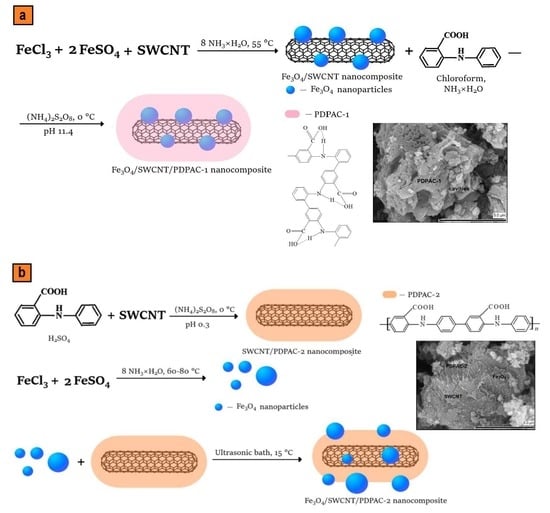
2.2. Synthesis of Fe3O4/SWCNT
2.3. Preparation of Fe3O4/SWCNT/PDPAC Nanocomposites
2.3.1. Preparation of Fe3O4/SWCNT/PDPAC Nanocomposites in the Interfacial Process in an Alkaline Medium
2.3.2. Preparation of Fe3O4/SWCNT/PDPAC Nanocomposites in an Acid Medium
2.4. Preparation of Suspensions for Magnetic Fluids
2.5. Characterization
3. Results and Discussion
3.1. Synthesis and Characterization of Nanomaterials
3.2. Electrical Characterization of Nanomaterials
- σdc—the frequency independent (dc) part of conductivity,
- n—the exponential parameter (0 ≤ n ≤ 1),
- A—the thermally activated quantity.
- A and n depend on the temperature and the volume fraction of the conducting component.
3.3. Thermal Properties of Nanomaterials
3.4. Magnetic Properties of Nanomaterials
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sellinger, A.; Weiss, P.M.; Nguyen, A.; Lu, Y.; Assink, R.A.; Gong, W.; Brinker, C.J. Continuous self-assembly of organic–inorganic nanocomposite coatings that mimic nacre. Nature 1998, 394, 256–259. [Google Scholar] [CrossRef]
- Yamamoto, K.; Sakata, Y.; Nohara, Y.; Takashi, Y.; Tatsumi, T. Organic-inorganic hybrid zeolites containing organic frameworks. Science 2003, 300, 470–472. [Google Scholar] [CrossRef]
- Balazs, A.C.; Emrick, T.; Russel, T.P. Nanoparticle polymer composites: Where two small worlds meet. Science 2006, 314, 1107–1110. [Google Scholar] [CrossRef]
- Karpacheva, G.P. Hybrid magnetic nanocomposites including polyconjugated polymers. Polym. Sci. C 2016, 58, 131–146. [Google Scholar]
- Zheng, Y.; Wang, X.; Wu, G. Chemical modification of carbon fiber with diethylenetriaminepentaacetic acid/halloysite nanotube as a multifunctional interfacial reinforcement for silicone resin composites. Polym. Adv. Technol. 2020, 31, 527–535. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, L.; Wang, X.; Wu, G. Modification of renewable cardanol onto carbon fiber for the improved interfacial properties of advanced polymer composites. Polymers 2020, 12, 45. [Google Scholar] [CrossRef] [Green Version]
- Godovsky, D.Y. Device applications of polymer-nanocomposites. Adv. Polym. Sci. 2000, 153, 163–205. [Google Scholar]
- Gerasin, V.A.; Antipov, E.M.; Karbushev, V.V.; Kulichikhin, V.G.; Karpacheva, G.P.; Talroze, R.V.; Kudryavtsev, Y.V. New approaches to the development of hybrid nanocomposites: From structural materials to high-tech applications. Russ. Chem. Rev. 2013, 82, 303–332. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Liu, S.; Li, L.; Zhang, C.; Liu, T. Conducting polymer composites: Material synthesis and applications in electrochemical capacitive energy storage. Mater. Chem. Front. 2017, 1, 251–268. [Google Scholar] [CrossRef]
- Lyu, L.; Liu, J.; Liu, H.; Liu, C.; Lu, Y.; Sun, K.; Fan, R.; Wang, N.; Guo, Z.; Wujcik, E.K. An overview of electrically conductive polymer nanocomposites toward electromagnetic interference shielding. Eng. Sci. 2018, 2, 26–42. [Google Scholar]
- Zhao, B.; Deng, J.; Zhang, R.; Liang, L.; Fan, B.; Bai, Z.; Shao, G.; Park, C.B. Recent advances on the electromagnetic wave absorption properties of Ni based materials. Eng. Sci. 2018, 3, 5–40. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Chen, Q.; Yang, S.; Lu, C.; Feng, M.; Jiang, Y.; Cao, G.; Zhang, J.; Liu, C. Micro-crack behavior of carbon fiber reinforced Fe3O4/graphene oxide modified epoxy composites for cryogenic application. Compos. Part A Appl. Sci. Manuf. 2018, 108, 12–22. [Google Scholar] [CrossRef]
- Wang, C.; Murugadoss, V.; Kong, J.; He, Z.; Mai, X.; Shao, Q.; Chen, Y.; Guo, L.; Liu, C.; Angaiah, S.; et al. Overview of carbon nanostructures and nanocomposites for electromagnetic wave shielding. Carbon 2018, 140, 696–733. [Google Scholar] [CrossRef]
- Chen, J.; Yu, Q.; Cui, X.; Dong, M.; Zhang, J.; Wang, C.; Fan, J.; Zhu, Y.; Guo, Z. An overview of stretchable strain sensors from conductive polymer nanocomposites. J. Mater. Chem. C 2019, 7, 11710–11730. [Google Scholar] [CrossRef]
- Wen, N.; Jiang, B.; Wang, X.; Shang, Z.; Jiang, D.; Zhang, L.; Sun, C.; Wu, Z.; Yan, H.; Liu, C.; et al. Overview of polyvinyl alcohol nanocomposite hydrogels for electro-skin, actuator, supercapacitor and fuel cell. Chem. Rec. 2020. [Google Scholar] [CrossRef]
- Wei, H.; Wang, H.; Cui, D.; Zhao, Z.; Chu, L.; Wei, X.; Wang, L.; Pan, D.; Fan, J.; Li, Y.; et al. Multifunctions of polymer nanocomposites: Environmental remediation, electromagnetic interference shielding, and sensing applications. ChemNanoMat 2020, 6, 174–184. [Google Scholar] [CrossRef]
- Zhang, L.; Du, W.Y.; Nautiyal, A.; Liu, Z.; Zhang, X.Y. Recent progress on nanostructured conducting polymers and composites: Synthesis, application and future aspects. Sci. China Mater. 2018, 61, 303–352. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.W.; Murugadoss, V.; Wang, Y.; Lin, J.; Ding, T.; Wang, Z.C.; Shao, Q.; Wang, C.; Liu, H.; Lu, N. Electromagnetic interference shielding polymers and nanocomposites—A Review. Polym. Rev. 2019, 59, 280–337. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Lin, Y.W.; Chang, C.C.; Wu, T.M. Conducting and magnetic behaviors of polyaniline coated multi-walled carbon nanotube composites containing monodispersed magnetite nanoparticles. Synth. Met. 2011, 161, 937–942. [Google Scholar] [CrossRef]
- Chen, T.; Qiu, J.; Zhu, K.; Che, Y.; Zhang, Y.; Zhang, J.; Li, H.; Wang, F.; Wang, Z. Enhanced electromagnetic wave absorption properties of polyaniline-coated Fe3O4/reduced graphene oxide nanocomposites. J. Mater. Sci. Mater. Electron. 2014, 25, 3664–3673. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, X.; Yu, K.; Zhang, C.; Li, H.; Du, Z. Preparation of multi-walled carbon nanotube/polyaniline/Fe3O4 composites. Integr. Ferroelectr. 2014, 154, 159–165. [Google Scholar] [CrossRef]
- Liu, P.; Huang, Y.; Zhang, X. Superparamagnetic Fe3O4 nanoparticles on graphene–polyaniline: Synthesis, characterization and their excellent electromagnetic absorption properties. J. Alloys Compd. 2014, 596, 25–31. [Google Scholar] [CrossRef]
- Wu, T.M.; Yen, S.J.; Chen, E.C.; Chiang, R.K. Synthesis, characterization, and properties of monodispersed magnetite coated multi-walled carbon nanotube/polypyrrole nanocomposites synthesized by in-situ chemical oxidative polymerization. J. Polym. Sci. B Polym. Phys. 2008, 46, 727–733. [Google Scholar] [CrossRef]
- He, Z.; Fang, Y.; Wang, X.; Pang, H. Microwave absorption properties of PANI/CIP/Fe3O4 composites. Synth. Met. 2011, 161, 420–425. [Google Scholar] [CrossRef]
- Liu, P.; Huang, Y.; Wang, L.; Zhang, W. Preparation and excellent microwave absoption property of three component nanocompostes: Polyaniline-reduced graphene oxide—Co3O4 nanoparticles. Synth. Met. 2013, 177, 89–93. [Google Scholar] [CrossRef]
- Yang, R.B.; Reddy, P.M.; Chang, C.J.; Chen, P.A.; Chen, J.K.; Chang, C.C. Synthesis and characterization of Fe3O4/polypyrrole/carbon nanotube composites with tunable microwave absorption properties: Role of carbon nanotube and polypyrrole content. Chem. Eng. J. 2016, 285, 497–507. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, Y.; Sun, Y.; Chen, H.; Xiong, Z.; Li, X.; Shen, L.; Liu, Y. Tunable magnetic properties of Fe3O4/rGO/PANI nanocomposites for enhancing microwave absorption performance. J. Alloys Compd. 2019, 796, 120–130. [Google Scholar] [CrossRef]
- Wang, S.; Bao, H.; Yang, P.; Chen, G. Immobilization of trypsin in polyaniline-coated nano-Fe3O4/carbon nanotube composite for protein digestion. Anal. Chim. Acta 2008, 612, 182–189. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Krishnamoorthy, K.; Sekar, C.; Wilson, J.; Kim, S.J. A promising sensing platform on ternary composite of polyaniline-Fe2O3-reduced graphene oxide for sensitive hydroquinone determination. Chem. Eng. J. 2015, 259, 594–602. [Google Scholar] [CrossRef]
- Giri, S.; Ghosh, D.; Das, C.K. In situ synthesis of cobalt doped polyaniline modified graphene composites for high performance supercapacitor electrode materials. J. Electroanal. Chem. 2013, 697, 32–45. [Google Scholar] [CrossRef]
- Xiong, P.; Huang, H.; Wang, X. Design and synthesis of ternary cobalt ferrite/graphene/polyaniline hierarchical nanocomposites for high-perfomance supercapacitors. J. Power Sources 2014, 245, 937–946. [Google Scholar] [CrossRef]
- Lin, H.; Huang, Q.; Wang, J.; Jiang, J.; Liu, F.; Chen, Y.; Wang, C.; Lu, D.; Han, S. Self-assembled graphene/polyaniline/Co3O4 ternary hybrid aerogels for supercapacitors. Electrochim. Acta 2016, 191, 444–451. [Google Scholar] [CrossRef]
- Ren, G.; Li, Y.; Guo, Z.; Xiao, G.; Zhu, Y.; Dai, L.; Jiang, L. A bio-inspired Co3O4-polypyrrole-graphene complex as an efficient oxygen reduction catalyst in one-step ball milling. Nano Res. 2015, 8, 3461–3471. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Li, F.; Li, W.; Yang, H.; Zhang, X.; Liu, Y.; Ma, J. A facile preparation of CoFe2O4 nanoparticles on polyaniline-functionalised carbon nanotubes as enhanced catalysts for the oxygen evolution reaction. J. Mater. Chem. A 2016, 4, 4472–4478. [Google Scholar] [CrossRef]
- Zhao, C.; Jin, Y.; Du, X.; Du, W. In situ prepared amorphous FeCoO- polyaniline/multiwalled carbon nanotube nanohybrids as efficient oxygen evolution catalysts for rechargeable Zn-air batteries. J. Power. Sources 2018, 399, 337–342. [Google Scholar] [CrossRef]
- Zhu, A.; Shi, P.; Sun, S.; Rui, M. Construction of rGO/Fe3O4/PANI nanocomposites and its corrosion resistance mechanism in waterborne acrylate-amino coating. Prog. Org. Coat. 2019, 133, 117–124. [Google Scholar] [CrossRef]
- Singh, A.P.; Mishra, M.; Sambyal, P.; Gupta, B.K.; Singh, B.P.; Chandra, A.; Dhawan, S.K. Encapsulation of γ-Fe2O3 decorated reduced graphene oxide in polyaniline core–shell tubes as an exceptional tracker for electromagnetic environmental pollution. J. Mater. Chem. A 2014, 2, 3581–3593. [Google Scholar] [CrossRef]
- Ibrahim, A.; Abdel-Aziz, M.H.; Zorombam, M.S.; Al-Hossainy, A.F. Structural, optical, and electrical properties of multi-walled carbon nanotubes/polyaniline/Fe3O4 ternary nanocomposites thin film. Synth. Met. 2018, 238, 1–13. [Google Scholar] [CrossRef]
- Kong, L.; Lu, X.; Zhang, W. Facile synthesis of multifunctional multiwalled carbon nanotubes/Fe3O4 nanoparticles/polyaniline composite nanotubes. J. Solid State Chem. 2008, 181, 628–636. [Google Scholar] [CrossRef]
- Cao, M.S.; Yang, J.; Song, W.L.; Zhang, D.Q.; Wen, B.; Jin, H.B.; Hou, Z.L.; Yuan, J. Ferroferric oxide/multiwalled carbon nanotube vs polyaniline/ferroferric oxide/multiwalled carbon nanotube multiheterostructures for highly effective microwave absorption. ACS Appl. Mater. Interfaces 2012, 4, 6949–6956. [Google Scholar] [CrossRef]
- Dai, L.; Mau, A.V.H. Controlled synthesis and modification of carbon nanotubes and C60: Carbon nanostructures for advanced polymeric composite materials. Adv. Mater. 2001, 13, 899–913. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon nanotubes—The route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [Green Version]
- Ozkan, S.Z.; Karpacheva, G.P.; Chernavskii, P.A.; Dzidziguri, E.L.; Bondarenko, G.N.; Pankina, G.V. Hybrid materials based on poly-3-amine-7-methylamine-2-methylphenazine and magnetite nanoparticles immobilized on single-walled carbon nanotubes. Polymers 2018, 10, 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozkan, S.Z.; Eremeev, I.S.; Karpacheva, G.P.; Bondarenko, G.N. Oxidative polymerization of N-phenylanthranilic acid in the heterophase system. Open J. Polym. Chem. 2013, 3, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Ozkan, S.Z.; Bondarenko, G.N.; Karpacheva, G.P. Oxidative polymerization of diphenylamine-2-carboxylic acid: Synthesis, structure, and properties of polymers. Polym. Sci. B 2010, 52, 263–269. [Google Scholar] [CrossRef]
- Ozkan, S.Z.; Eremeev, I.S.; Karpacheva, G.P.; Prudskova, T.N.; Veselova, E.V.; Bondarenko, G.N.; .Shandryuk, G.A. Polymers of dipheylamine-2-carboxylic acid: Synthesis, structure and properties. Polym. Sci. B 2013, 55, 107–115. [Google Scholar]
- Dzidziguri, E.L. Dimensional characteristics of nanopowders. Nanotechnologies Russ. 2009, 4, 857–870. [Google Scholar] [CrossRef]
- Chernavskii, P.A.; Pankina, G.V.; Lunin, V.V. Magnetometric methods of investigation of supported catalysts. Russ. Chem. Rev. 2011, 80, 579–604. [Google Scholar] [CrossRef]
- Soloveva, A.Y.; Ioni, Y.V.; Gubin, S.P. Synthesis of Fe3O4 nanoparticles on the surface of graphene. Mendeleev Commun. 2016, 26, 38–39. [Google Scholar] [CrossRef]
- Abrosimova, G.E.; Aronin, A.S.; Kholstinina, N.N. On the determination of the volume fraction of the crystalline phase in amorphous-crystalline alloys. Phys. Solid State 2010, 52, 445–451. [Google Scholar] [CrossRef]
- Ozkan, S.Z.; Karpacheva, G.P.; Kostev, A.I.; Bondarenko, G.N. Formation features of hybrid nanocomposites based on polydiphenylamine-2-carboxylic acid and single-walled carbon nanotubes. Polymers 2019, 11, 1181. [Google Scholar] [CrossRef] [Green Version]
- Zengin, H.; Zhou, W.; Jin, J.; Czerw, R.; Smith, D.W., Jr.; Echegoyen, L.; Carroll, D.L.; Foulger, S.H.; Ballato, J. Carbon nanotube doped polyaniline. Adv. Mater. 2002, 14, 1480–1483. [Google Scholar] [CrossRef]
- Cochet, M.; Maser, W.K.; Benito, A.M.; Callejas, M.A.; Martínez, M.T.; Benoit, J.-M.; Schreiber, J.; Chauvet, O. Synthesis of a new polyaniline/nanotube composite: “in-situ” polymerisation and charge transfer through site-selective interaction. Chem. Commun. 2001, 37, 1450–1451. [Google Scholar] [CrossRef]
- Xu, J.; Yao, P.; Liu, L.; Jiang, Z.; He, F.; Li, M.; Zou, J. Synthesis and characterization of an organic soluble and conducting polyaniline-grafted multiwalled carbon nanotube core–shell nanocomposites by emulsion polymerization. J. Appl. Polym. Sci. 2010, 118, 2582–2591. [Google Scholar] [CrossRef]
- Jonscher, A.K. The ‘universal’ dielectric response. Nature 1977, 267, 673–679. [Google Scholar] [CrossRef]
- Dyre, J.C.; Schrøder, T.B. Universality of ac conduction in disordered solids. Rev. Mod. Phys. 1999, 72, 873–892. [Google Scholar] [CrossRef] [Green Version]
- Xie, P.; Li, Y.; Hou, Q.; Sui, K.; Liu, C.; Fu, X.; Zhang, J.; Murugadoss, V.; Fan, J.; Wang, Y.; et al. Tunneling-induced negative permittivity in Ni/MnO nanocomposites by a bio-gel derived strategy. J. Mater. Chem. C. 2020, 8, 3029–3039. [Google Scholar] [CrossRef]
- Rehwald, W.; Kiess, H.; Binggeli, B. Frequency dependent conductivity in polymers and other disordered materials. Z. Phys. B Condens. Matter. 1987, 68, 143–148. [Google Scholar] [CrossRef]
- Dyre, J.C. The random free-energy barrier model for ac conduction in disordered solids. J. Appl. Phys. 1988, 64, 2456–2468. [Google Scholar] [CrossRef] [Green Version]
- Gubin, S.P.; Koksharov, Y.A.; Khomutov, G.B.; Yurkov, G.Y. Magnetic nanoparticles: Preparation, structure and properties. Russ. Chem. Rev. 2005, 7, 489–520. [Google Scholar] [CrossRef]
















| Nanomaterials Abbreviations | Fe, % * | Preparation Method | ** Amount of SWCNT, g | Amount of DPAC, g | *** Amount of Iron Salts, g | |
|---|---|---|---|---|---|---|
| Fe (II) | Fe (III) | |||||
| F34C3 **** | 61.2 | Synthesis of Fe3O4/SWCNT in an alkaline medium | 0.03 | 0 | 0.86 | 2.35 |
| F34C3P-1 | 33.5 | Polymerization of DPAC in the interfacial process in an alkaline medium with Fe3O4/SWCNT | 0.03 | 1.0 | 0.86 | 2.35 |
| F18C3P-1 | 17.9 | 0.43 | 1.18 | |||
| F14C3P-1 | 12.0 | 0.22 | 0.59 | |||
| F7C3P-1 | 6.4 | 0.11 | 0.29 | |||
| F7C10P-1 | 8.4 | 0.1 | ||||
| F34C3P-2 | 34.7 | Precipitation of Fe3O4 onto the surface of the SWCNT/PDPAC, prepared in an acidic medium | 0.019 | 0.64 | 0.43 | 1.18 |
| F18C3P-2 | 17.8 | 0.27 | 0.73 | |||
| F14C3P-2 | 13.4 | 0.11 | 0.29 | |||
| F7C3P-2 | 7.6 | 0.054 | 0.15 | |||
| F7C10P-2 | 6.2 | 0.064 | ||||
| Materials | Volume Fraction, % | ||
|---|---|---|---|
| Amorphous Polymer | Crystalline Polymer | Fe3O4 | |
| PDPAC-1 | 100.00 | - | - |
| F14C3P-1 | 37.69 | - | 62.31 |
| F34C3P-1 | 5.24 | - | 94.76 |
| F34C3 * | - | - | 100.00 |
| PDPAC-2 | 95.60 | 4.40 | - |
| F14C3P-2 | 28.99 | 2.35 | 68.66 |
| F34C3P-2 | 6.21 | 0.75 | 93.05 |
| Materials | * σac, S/cm | σdc, S/cm | n | A | |
|---|---|---|---|---|---|
| PDPAC-1 | 3.1 × 10−12 | 1.1 × 10−7 | 2.8 × 10−12 | 0.75 | 8.5 × 10−12 |
| F7C3P-1 | 3.6 × 10−10 | 1.2 × 10−7 | 2.4 × 10−10 | 0.82 | 1.7 × 10−13 |
| F7C10P-1 | 1.2 × 10−8 | 9.6 × 10−7 | 1.1 × 10−8 | 0.96 | 1.9 × 10−12 |
| F34C3P-1 | 8.1 × 10−9 | 8.7 × 10−7 | 6.8 × 10−9 | 0.99 | 5.4 × 10−13 |
| F34C3 ** | 3.5 × 10−9 | 2.5 × 10−6 | 2.3 × 10−9 | 1.00 | 9.7 × 10−13 |
| PDPAC-2 | 1.4 × 10−5 | 2.2 × 10−5 | 1.0 × 10−5 | 0.45 | 6.9 × 10−9 |
| F7C3P-2 | 2.5 × 10−4 | 2.7 × 10−4 | 2.1 × 10−4 | 0.31 | 3.0 × 10−7 |
| F7C10P-2 | 3.7 × 10−3 | 3.3 × 10−3 | 3.2 × 10−3 | 0.51 | 2.0 × 10−8 |
| F14C3P-2 | 4.6 × 10−6 | 3.1 × 10−5 | 4.3 × 10−6 | 0.64 | 9.0 × 10−11 |
| F34C3P-2 | 3.4 × 10−7 | 3.6 × 10−6 | 3.1 × 10−7 | 0.74 | 1.3 × 10−9 |
| Materials | * T5%, °C | ** T25%, °C | *** T50%, °C | **** Residue, % |
|---|---|---|---|---|
| PDPAC-1 | 182/205 | 400/324 | 522/663 | 0/19 |
| PDPAC-2 | 104/102 | 232/243 | 517/396 | 0/35 |
| F34C3P-1 | 238/201 | 403/628 | > 1000/910 | 53/48 |
| F34C3P-2 | 180/225 | 388/677 | 542/ > 1000 | 48/52 |
| Nanomaterials | Fe, % * | HC, Oe | MS, emu/g | MR, emu/g | MR/MS |
|---|---|---|---|---|---|
| F7C3P-1 | 6.4 | 0 | 4.8 | 0 | 0 |
| F10C3P-1 | 8.4 | 0 | 11.3 | 0 | 0 |
| F14C3P-1 | 12.0 | 0 | 15.7 | 0 | 0 |
| F18C3P-1 | 17.9 | 0 | 22.5 | 0 | 0 |
| F34C3P-1 | 33.5 | 0 | 31.6 | 0 | 0 |
| F34C3 ** | 61.2 | 6 | 47.3 | 0.45 | 0.009 |
| F7C3P-2 | 7.6 | 0 | 5.3 | 0 | 0 |
| F7C10P-2 | 6.2 | 0 | 4.5 | 0 | 0 |
| F14C3P-2 | 13.4 | 0 | 12.1 | 0 | 0 |
| F18C3P-2 | 17.8 | 0 | 26.1 | 0 | 0 |
| F34C3P-2 | 34.7 | 0 | 39.4 | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozkan, S.Z.; Kostev, A.I.; Karpacheva, G.P.; Chernavskii, P.A.; Vasilev, A.A.; Muratov, D.G. Hybrid Electromagnetic Nanomaterials Based on Polydiphenylamine-2-carboxylic Acid. Polymers 2020, 12, 1568. https://doi.org/10.3390/polym12071568
Ozkan SZ, Kostev AI, Karpacheva GP, Chernavskii PA, Vasilev AA, Muratov DG. Hybrid Electromagnetic Nanomaterials Based on Polydiphenylamine-2-carboxylic Acid. Polymers. 2020; 12(7):1568. https://doi.org/10.3390/polym12071568
Chicago/Turabian StyleOzkan, Sveta Zhiraslanovna, Aleksandr Ivanovich Kostev, Galina Petrovna Karpacheva, Petr Aleksandrovich Chernavskii, Andrey Aleksandrovich Vasilev, and Dmitriy Gennad’evich Muratov. 2020. "Hybrid Electromagnetic Nanomaterials Based on Polydiphenylamine-2-carboxylic Acid" Polymers 12, no. 7: 1568. https://doi.org/10.3390/polym12071568
APA StyleOzkan, S. Z., Kostev, A. I., Karpacheva, G. P., Chernavskii, P. A., Vasilev, A. A., & Muratov, D. G. (2020). Hybrid Electromagnetic Nanomaterials Based on Polydiphenylamine-2-carboxylic Acid. Polymers, 12(7), 1568. https://doi.org/10.3390/polym12071568