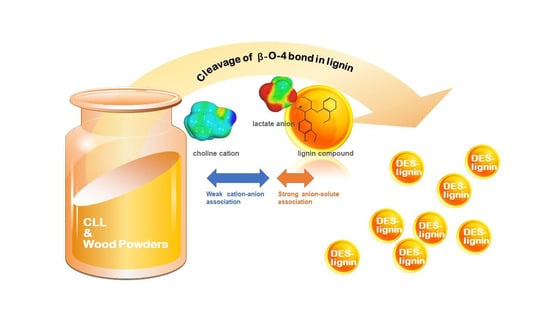
Enhancement of Lignin Extraction of Poplar by Treatment of Deep Eutectic Solvent with Low Halogen Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals
2.3. Preparation of DES
2.4. DES Treatment
2.5. Preparation of Milled Wood Lignin
2.6. Analysis of Lignin Characteristics
2.6.1. FT-IR Analysis
2.6.2. Acetylation of Lignin
2.6.3. GPC Analysis
2.6.4. HSQC NMR Analysis
3. Results and Discussion
3.1. Lignin Extracted by Choline-Based DES
3.2. Characterization of Lignin
3.2.1. Lignin Purity
3.2.2. Molecular Weight Distribution
3.3. Structure Characterization of Lignin
3.3.1. FT-IR Analysis
3.3.2. HSQC NMR Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elgharbawy, A.A.; Hayyan, M.; Hayyan, A.; Basirun, W.J.; Salleh, H.M.; Mirghani, M.E. A grand avenue to integrate deep eutectic solvents into biomass processing. Biomass Bioenergy 2020, 137, 105550. [Google Scholar] [CrossRef]
- Stafford, W.; De Lange, W.; Nahman, A.; Chunilall, V.; Lekha, P.; Andrew, J.; Johakimu, J.; Sithole, B.; Trotter, D. Forestry biorefineries. Renew. Energy 2020, 154, 461–475. [Google Scholar] [CrossRef]
- Stewart, D. Lignin as a base material for materials applications: Chemistry, application and economics. Ind. Crop. Prod. 2008, 27, 202–207. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Satari, B.; Karimi, K.; Kumar, R. Cellulose solvent-based pretreatment for enhanced second-generation biofuel production: A review. Sustain. Energy Fuels 2019, 3, 11–62. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Q.; You, T.; Zhang, X.; Xu, F.; Wu, Y.-Y. Short-time deep eutectic solvent pretreatment for enhanced enzymatic saccharification and lignin valorization. Green Chem. 2019, 21, 3099–3108. [Google Scholar] [CrossRef]
- Alvarez-Vasco, C.; Ma, R.; Quintero, M.; Guo, M.; Geleynse, S.; Ramasamy, K.K.; Wolcott, M.P.; Zhang, X. Unique low-molecular-weight lignin with high purity extracted from wood by deep eutectic solvents (DES): A source of lignin for valorization. Green Chem. 2016, 18, 5133–5141. [Google Scholar] [CrossRef]
- Li, T.; Lyu, G.; Liu, Y.; Lou, R.; Lucia, L.A.; Yang, G.; Chen, J.; Saeed, H.A. Deep Eutectic Solvents (DESs) for the Isolation of Willow Lignin (Salix matsudana cv. Zhuliu). Int. J. Mol. Sci. 2017, 18, 2266. [Google Scholar] [CrossRef] [Green Version]
- An, Y.-X.; Zong, M.-H.; Wu, H.; Li, N. Pretreatment of lignocellulosic biomass with renewable cholinium ionic liquids: Biomass fractionation, enzymatic digestion and ionic liquid reuse. Bioresour. Technol. 2015, 192, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.; Hu, C. Selective extraction and conversion of lignin in actual biomass to monophenols: A review. J. Energy Chem. 2016, 25, 947–956. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 278. [Google Scholar] [CrossRef] [Green Version]
- Al Arni, S. Extraction and isolation methods for lignin separation from sugarcane bagasse: A review. Ind. Crop. Prod. 2018, 115, 330–339. [Google Scholar] [CrossRef]
- Xu, H.; Peng, J.; Kong, Y.; Liu, Y.; Su, Z.; Li, B.; Tian, W. Key process parameters for deep eutectic solvents pretreatment of lignocellulosic biomass materials: A review. Bioresour. Technol. 2020, 123416. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixturesElectronic supplementary information (ESI) available: Spectroscopic data. Chem. Commun. 2003, 9, 70–71. Available online: http://www.rsc.org/suppdata/cc/b2/b210714g/ (accessed on 10 July 2020). [CrossRef] [PubMed] [Green Version]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Francisco, M.; Bruinhorst, A.V.D.; Kroon, M.C. New natural and renewable low transition temperature mixtures (LTTMs): Screening as solvents for lignocellulosic biomass processing. Green Chem. 2012, 14, 2153–2157. [Google Scholar] [CrossRef]
- Procentese, A.; Johnson, E.; Orr, V.; Campanile, A.G.; Wood, J.A.; Marzocchella, A.; Rehmann, L. Deep eutectic solvent pretreatment and subsequent saccharification of corncob. Bioresour. Technol. 2015, 192, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-W.; Xia, S.; Ma, P.-S. Facile pretreatment of lignocellulosic biomass using deep eutectic solvents. Bioresour. Technol. 2016, 219, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.K.; Parikh, B.S.; Pravakar, M. Natural deep eutectic solvent mediated pretreatment of rice straw: Bioanalytical characterization of lignin extract and enzymatic hydrolysis of pretreated biomass residue. Environ. Sci. Pollut. Res. 2015, 23, 9265–9275. [Google Scholar] [CrossRef] [PubMed]
- Brandt-Talbot, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef] [Green Version]
- Ninomiya, K.; Yamauchi, T.; Kobayashi, M.; Ogino, C.; Shimizu, N.; Takahashi, K. Cholinium carboxylate ionic liquids for pretreatment of lignocellulosic materials to enhance subsequent enzymatic saccharification. Biochem. Eng. J. 2013, 71, 25–29. [Google Scholar] [CrossRef]
- Ninomiya, K.; Inoue, K.; Aomori, Y.; Ohnishi, A.; Ogino, C.; Shimizu, N.; Takahashi, K. Characterization of fractionated biomass component and recovered ionic liquid during repeated process of cholinium ionic liquid-assisted pretreatment and fractionation. Chem. Eng. J. 2015, 259, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.-P.; Hou, X.-D.; Li, N.; Zong, M.-H. Ionic liquids from renewable biomaterials: Synthesis, characterization and application in the pretreatment of biomass. Green Chem. 2012, 14, 304–307. [Google Scholar] [CrossRef]
- Hou, X.-D.; Smith, T.J.; Li, N.; Zong, M.-H. Novel renewable ionic liquids as highly effective solvents for pretreatment of rice straw biomass by selective removal of lignin. Biotechnol. Bioeng. 2012, 109, 2484–2493. [Google Scholar] [CrossRef]
- Hou, X.-D.; Li, N.; Zong, M.-H. Facile and Simple Pretreatment of Sugar Cane Bagasse without Size Reduction Using Renewable Ionic Liquids–Water Mixtures. ACS Sustain. Chem. Eng. 2013, 1, 519–526. [Google Scholar] [CrossRef]
- Hou, X.-D.; Li, N.; Zong, M.-H. Significantly enhancing enzymatic hydrolysis of rice straw after pretreatment using renewable ionic liquid–water mixtures. Bioresour. Technol. 2013, 136, 469–474. [Google Scholar] [CrossRef]
- Hou, X.-D.; Xu, J.; Li, N.; Zong, M.-H. Effect of anion structures on cholinium ionic liquids pretreatment of rice straw and the subsequent enzymatic hydrolysis. Biotechnol. Bioeng. 2014, 112, 65–73. [Google Scholar] [CrossRef]
- Hou, X.-D.; Liu, Q.-P.; Smith, T.J.; Li, N.; Zong, M.-H. Evaluation of Toxicity and Biodegradability of Cholinium Amino Acids Ionic Liquids. PLoS ONE. 2013, 8, e59145. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.L.; Holbrey, J.D.; Ng, S.; Plechkova, N.V.; Seddon, K.R.; Tomaszowska, A.A.; Wassell, D.F. A greener, halide-free approach to ionic liquid synthesis. Pure Appl. Chem. 2011, 84, 723–744. [Google Scholar] [CrossRef]
- Muhammad, N.; Hossain, M.I.; Man, Z.; El-Harbawi, M.; Bustam, M.A.; Noaman, Y.A.; Alitheen, N.B.M.; Ng, M.K.; Hefter, G.; Yin, C.-Y. Synthesis and Physical Properties of Choline Carboxylate Ionic Liquids. J. Chem. Eng. Data. 2012, 57, 2191–2196. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Laboration Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Peng, J.; Yang, G.; Qi, L.; Liu, J.; Li, F.; Chen, J.; Lucia, L.A. Enhancement of Delignification by Ionic Liquids Pretreatment and Modification of Hardwood Kraft Pulp in Preparation for Bleaching. BioRes. 2020, 15, 6299–6308. [Google Scholar] [CrossRef]
- Björkman, A. Studies on finely divided wood. Part 1. Extraction of lignin with neutral solvents. Sven. Papperst 1956, 59, 477–485. [Google Scholar]
- Lyu, G.; Li, T.; Ji, X.; Yang, G.; Liu, Y.; Lucia, L.; Chen, J. Characterization of Lignin Extracted from Willow by Deep Eutectic Solvent Treatments. Polymers 2018, 10, 869. [Google Scholar] [CrossRef] [Green Version]
- Li, L.F.; Hu, Y.C. Application of ionic liquids in lignin processing. Chem. Ind. For. Prod. 2015, 35, 163–170. [Google Scholar] [CrossRef]
- Sun, Y.-C.; Xu, J.-K.; Xu, F.; Sun, S. Efficient separation and physico-chemical characterization of lignin from eucalyptus using ionic liquid–organic solvent and alkaline ethanol solvent. Ind. Crop. Prod. 2013, 47, 277–285. [Google Scholar] [CrossRef]
- Jääskeläinen, A.-S.; Liitiä, T.; Mikkelson, A.; Tamminen, T. Aqueous organic solvent fractionation as means to improve lignin homogeneity and purity. Ind. Crop. Prod. 2017, 103, 51–58. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Yoo, C.G.; Yang, X.; Lin, X.; Ralph, J.; Pan, X. An uncondensed lignin depolymerized in the solid state and isolated from lignocellulosic biomass: A mechanistic study. Green Chem. 2018, 20, 4224–4235. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Chen, J.; Wang, C.; Yang, G.; Ji, X.; Peng, J.; Xu, F. Quantitative structures and thermal properties of Miscanthus × giganteus lignin after alcoholamine-based ionic liquid pretreatment. Ind. Crop. Prod. 2020, 147, 112232. [Google Scholar] [CrossRef]
- Brandt, A.; Welton, T.; Chen, L.; Van Dongen, B.E.; Hallett, J.P. Structural changes in lignins isolated using an acidic ionic liquid water mixture. Green Chem. 2015, 17, 5019–5034. [Google Scholar] [CrossRef] [Green Version]
- Miles-Barrett, D.M.; Neal, A.R.; Hand, C.; Montgomery, J.R.D.; Panovic, I.; Ojo, O.S.; Lancefield, C.S.; Cordes, D.B.; Slawin, A.M.Z.; Lebl, T.; et al. The synthesis and analysis of lignin-bound Hibbert ketone structures in technical lignins. Org. Biomol. Chem. 2016, 14, 10023–10030. [Google Scholar] [CrossRef] [Green Version]
- De Gregorio, G.F.; Weber, C.C.; Gräsvik, J.; Welton, T.; Brandt, A.; Hallett, J.P. Mechanistic insights into lignin depolymerisation in acidic ionic liquids. Green Chem. 2016, 18, 5456–5465. [Google Scholar] [CrossRef]
- Jia, S.; Cox, B.J.; Guo, X.; Zhang, Z.C.; Ekerdt, J.G. Cleaving the β–O–4 Bonds of Lignin Model Compounds in an Acidic Ionic Liquid, 1-H-3-Methylimidazolium Chloride: An Optional Strategy for the Degradation of Lignin. ChemSusChem 2010, 3, 1078–1084. [Google Scholar] [CrossRef]





| DES | Tm (°C) | Viscosity (Pa·s) | Density (g/cm3) | pH |
|---|---|---|---|---|
| CCL | 18.34 ± 0.34 | 0.87 | 1.2022 | 0.91 |
| CLL | 18.14 ± 0.39 | 1.38 | 1.2078 | 2.75 |
| Glucose | Xylose | Mannose | Galactose | Arabinose | Lignin | |
|---|---|---|---|---|---|---|
| CCL solid residue | 95.29 ± 3.42 | 25.80 ± 1.11 | – | – | – | 9.73 ± 0.78 |
| CCL lignin | 0.17 ± 0.1 | – | – | – | – | 86.02 ± 1.58 |
| CLL solid residue | 98.40 ± 0.42 | 24.10 ± 1.15 | 67.01 ± 2.21 | – | – | 7.03 ± 0.58 |
| CLL lignin | 0.10 ± 0.1 | – | – | – | – | 90.13 ± 2.63 |
| Glucose | Xylose | Mannose | Galactose | Arabinose | ASL | AIL | Lignin | |
|---|---|---|---|---|---|---|---|---|
| Poplar | 45.46 ± 1.21 | 14.08 ± 0.37 | 1.71 ± 0.19 | 0.41 ± 0.10 | 0.21 ± 0.10 | 5.05 | 20.57 | 25.62 ± 0.32 |
| CCL lignin | 0.35 ± 0.10 | – | – | – | – | 2.30 | 86.52 | 88.82 ± 1.25 |
| CLL lignin | 0.21 ± 0.10 | – | – | – | – | 1.92 | 89.25 | 91.17 ± 2.15 |
| Mw (g/mol) | Mn (g/mol) | PDI | |
|---|---|---|---|
| Lit. MWL * | 10,000 | 4166 | 2.40 |
| MWL | 6374 | 5542 | 1.15 |
| CCL lignin | 4416 | 2349 | 1.88 |
| CLL lignin | 1805 | 971 | 1.86 |
| Wavenumbers (cm−1) | Assignment (Bond) | Initial Sample | CLL Lignin | CCL Lignin | MWL |
|---|---|---|---|---|---|
| 3420 | O–H stretching vibration | √ | √ | √ | √ |
| 2930 | C–H stretching vibration in methyl | √ | √ | √ | √ |
| 1710 | C=O stretching vibration | √ | √ | √ | √ |
| 1604,1510 | Aromatic ring skeleton vibration | √ | √ | √ | √ |
| 1460 | C–H deformation vibration in -CH2- | √ | √ | √ | √ |
| 1373 | C–H bending vibration of aliphatic compounds in carbohydrate | √ | × | × | × |
| 1328,1220 | C–O stretching vibration of syringyl units | √ | √ | √ | √ |
| 1270 | C–O stretching vibration of guaiacyl units | √ | √ | √ | √ |
| 1162 | C–O–C symmetrical stretching vibration in carbohydrate | √ | × | × | × |
| 1116 | C–H stretching vibration of syringyl units | √ | √ | √ | √ |
| 1032 | C–H bending vibration of guaiacyl units | √ | √ | √ | √ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Qi, L.; Yang, G.; Xue, Y.; He, M.; Lucia, L.A.; Chen, J. Enhancement of Lignin Extraction of Poplar by Treatment of Deep Eutectic Solvent with Low Halogen Content. Polymers 2020, 12, 1599. https://doi.org/10.3390/polym12071599
Liu J, Qi L, Yang G, Xue Y, He M, Lucia LA, Chen J. Enhancement of Lignin Extraction of Poplar by Treatment of Deep Eutectic Solvent with Low Halogen Content. Polymers. 2020; 12(7):1599. https://doi.org/10.3390/polym12071599
Chicago/Turabian StyleLiu, Jinke, Letian Qi, Guihua Yang, Yu Xue, Ming He, Lucian A. Lucia, and Jiachuan Chen. 2020. "Enhancement of Lignin Extraction of Poplar by Treatment of Deep Eutectic Solvent with Low Halogen Content" Polymers 12, no. 7: 1599. https://doi.org/10.3390/polym12071599
APA StyleLiu, J., Qi, L., Yang, G., Xue, Y., He, M., Lucia, L. A., & Chen, J. (2020). Enhancement of Lignin Extraction of Poplar by Treatment of Deep Eutectic Solvent with Low Halogen Content. Polymers, 12(7), 1599. https://doi.org/10.3390/polym12071599