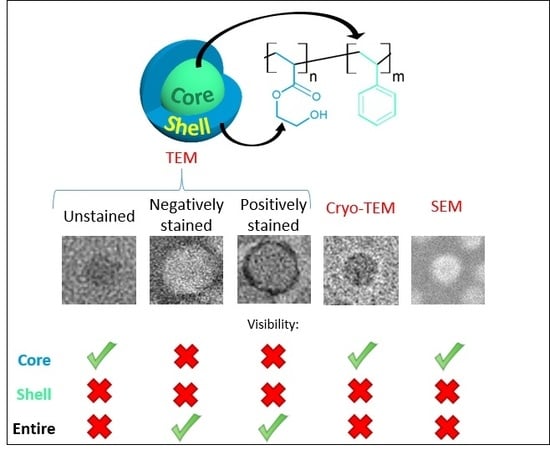
Characterizing the Core-Shell Architecture of Block Copolymer Nanoparticles with Electron Microscopy: A Multi-Technique Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
- -
- For dried samples, latex samples were diluted 200 times with a methanol/water mixture (to get a concentration of 0.1 wt %), cast onto the TEM grid, and dried overnight at room temperature.
- -
- For positive staining TEM, ruthenium tetroxide (RuO4) vapors was produced in situ by reacting 0.5 mL of a 13 wt % aqueous solution of sodium hypochlorite with 150 mg of RuCl3∙3H2O in a 10-cm diameter petri dish. A TEM grid with the disposed sample (the preparation of the grid according to the same procedure as for dried samples/conventional TEM) was placed close to the reaction mixture, and a second petri dish of similar size was positioned above to form a closed chamber. The grid was left for 10 min. A scheme of the experimental set-up is available in the SI (Figure S2) [31].
- -
- For negative staining TEM, a rapid flushing method was implemented. The protocol was originally developed by Imai et al. [32] and more recently adapted by Scarff et al. [33]. The idea of the method is to minimize the time the sample has to interact with the support of the grid surface before fixation. The goal is to hinder structural changes in the specimen that could occur upon prolonged absorption time on the carbon film or through capillary action. Before sample application, the TEM grid was faced upon a microscope slide and then irradiated in a glow discharge unit (UV/Ozone ProCleaner Plus) for a minimum of 300 s to render it hydrophilic. As in a typical procedure, 70 µL of uranyl acetate (1 wt % solution in water) was drawn up into the tip of a 200 µL pipette; 10 µL of the air gap were subsequently drawn up, then a 20 µL of deionized water (acting as wash/mixing agent) followed by another air gap of 10 µL, and finally 10 µL of sample solution (1 wt %). The edge of the grid was gripped with a pair of negative pressure tweezers, holding the tweezers so that the grid was angled at approximately 45° facing away from the researcher. The entire content of the pipette tip was ejected across the face of the TEM grid. The excess of stain was removed by touching the torn edge of a piece of filter paper to the edge of the grid. The grid was left to dry over air. Due to difficulties to efficiently adsorb PHEA23–b–PS130-based nanoparticles with the latter protocol, preparation conditions were changed: a 5 µL drop of ethanol was applied onto 400 mesh Cu grids covered with a plain carbon film. After a 1 min interaction, the excess was removed and a 5 µL drop of latex (0.1 wt %) was applied. After 1 min, the excess was removed by touching the torn edge of a piece of filter paper to the edge of the grid, and a 5 µL drop of 2 wt % uranyl acetate aqueous solution was immediately added. After 1 min, the grid was fully dried with a piece of filter paper, and a Tecnai G2 microscope (FEI) operating at 200 kV was used for the imaging of this PHEA23–b–PS130 sample.
3. Results and Discussion
3.1. Synthesis of Amphiphilic Diblock Copolymer Nanoparticles PHEA-b-PS
3.2. Conventional TEM
3.2.1. Unstained Samples
3.2.2. Positive Staining
3.2.3. Negative Staining
3.3. Cryo-TEM
3.4. SEM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Letchford, K.; Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Yun, Y.H.; Park, K. Smart nanoparticles for drug delivery: Boundaries and opportunities. Chem. Eng. Sci. 2015, 125, 158–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolai, T.; Colombani, O.; Chassenieux, C. Dynamic polymeric micelles versus frozen nanoparticles formed by block copolymers. Soft Matter 2010, 6, 3111–3118. [Google Scholar] [CrossRef]
- Zehm, D.; Ratcliffe, L.P.D.; Armes, S.P. Synthesis of Diblock Copolymer Nanoparticles via RAFT Alcoholic Dispersion Polymerization: Effect of Block Copolymer Composition, Molecular Weight, Copolymer Concentration, and Solvent Type on the Final Particle Morphology. Macromolecules 2013, 46, 128–139. [Google Scholar] [CrossRef]
- Le, D.; Keller, D.; Delaittre, G. Reactive and Functional Nanoobjects by Polymerization-Induced Self-Assembly. Macromol. Rapid Commun. 2019, 40, 1800551. [Google Scholar] [CrossRef]
- Blackman, L.D.; Varlas, S.; Arno, M.C.; Houston, Z.H.; Fletcher, N.L.; Thurecht, K.J.; Hasan, M.; Gibson, M.I.; O’Reilly, R.K. Confinement of Therapeutic Enzymes in Selectively Permeable Polymer Vesicles by Polymerization-Induced Self-Assembly (PISA) Reduces Antibody Binding and Proteolytic Susceptibility. ACS Cent. Sci. 2018, 4, 718–723. [Google Scholar] [CrossRef]
- Xiong, X.-B.; Falamarzian, A.; Garg, S.M.; Lavasanifar, A. Engineering of amphiphilic block copolymers for polymeric micellar drug and gene delivery. J. Control. Release 2011, 155, 248–261. [Google Scholar] [CrossRef]
- Reisch, A.; Klymchenko, A.S. Fluorescent Polymer Nanoparticles Based on Dyes: Seeking Brighter Tools for Bioimaging. Small 2016, 12, 1968–1992. [Google Scholar] [CrossRef] [Green Version]
- Chenal, M.; Rieger, J.; Véchambre, C.; Chenal, J.-M.; Chazeau, L.; Creton, C.; Bouteiller, L. Soft Nanostructured Films with an Ultra-Low Volume Fraction of Percolating Hard Phase. Macromol. Rapid Commun. 2013, 34, 1524–1529. [Google Scholar] [CrossRef]
- Cayre, O.J.; Chagneux, N.; Biggs, S. Stimulus responsive core-shell nanoparticles: Synthesis and applications of polymer based aqueous systems. Soft Matter 2011, 7, 2211–2234. [Google Scholar] [CrossRef]
- Bertrand, O.; Gohy, J.-F. Photo-responsive polymers: Synthesis and applications. Polym. Chem. 2017, 8, 52–73. [Google Scholar] [CrossRef]
- Tkachenko, V.; Matei Ghimbeu, C.; Vaulot, C.; Vidal, L.; Poly, J.; Chemtob, A. RAFT-photomediated PISA in dispersion: Mechanism, optical properties and application in templated synthesis. Polym. Chem. 2019, 10, 2316–2326. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Matyjaszewski, K.; Pietrasik, J. Versatile PISA templates for tailored synthesis of nanoparticles. Eur. Polym. J. 2019, 110, 49–55. [Google Scholar] [CrossRef]
- Musyanovych, A.; Landfester, K. Core–Shell Particles. In Macromolecular Engineering; John Wiley & Sons, Ltd.: Weinheim, Germany, 2011; pp. 1209–1247. ISBN 978-3-527-63142-1. [Google Scholar]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Berezin, M.Y.; Fan, J.; Lee, H.; Ma, J.; Zhang, K.; Wooley, K.L.; Achilefu, S. Bright fluorescent nanoparticles for developing potential optical imaging contrast agents. Nanoscale 2010, 2, 548–558. [Google Scholar] [CrossRef] [Green Version]
- Keller, D.; Beloqui, A.; Martínez-Martínez, M.; Ferrer, M.; Delaittre, G. Nitrilotriacetic Amine-Functionalized Polymeric Core–Shell Nanoparticles as Enzyme Immobilization Supports. Biomacromolecules 2017, 18, 2777–2788. [Google Scholar] [CrossRef]
- Ladmiral, V.; Semsarilar, M.; Canton, I.; Armes, S.P. Polymerization-Induced Self-Assembly of Galactose-Functionalized Biocompatible Diblock Copolymers for Intracellular Delivery. J. Am. Chem. Soc. 2013, 135, 13574–13581. [Google Scholar] [CrossRef]
- Chenal, M.; Véchambre, C.; Chenal, J.-M.; Chazeau, L.; Humblot, V.; Bouteiller, L.; Creton, C.; Rieger, J. Mechanical properties of nanostructured films with an ultralow volume fraction of hard phase. Polymer 2017, 109, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Gosecka, M.; Gosecki, M. Characterization methods of polymer core–shell particles. Colloid Polym. Sci. 2015, 293, 2719–2740. [Google Scholar] [CrossRef]
- Fenyves, R.; Schmutz, M.; Horner, I.J.; Bright, F.V.; Rzayev, J. Aqueous Self-Assembly of Giant Bottlebrush Block Copolymer Surfactants as Shape-Tunable Building Blocks. J. Am. Chem. Soc. 2014, 136, 7762–7770. [Google Scholar] [CrossRef]
- Müllner, M.; Müller, A.H.E. Cylindrical polymer brushes—Anisotropic building blocks, unimolecular templates and particulate nanocarriers. Polymer 2016, 98, 389–401. [Google Scholar] [CrossRef]
- Gröschel, A.H.; Müller, A.H.E. Self-assembly concepts for multicompartment nanostructures. Nanoscale 2015, 7, 11841–11876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Z.; Gourevich, I.; Field, L.; Coombs, N.; Kumacheva, E. TEM Imaging of Polymer Multilayer Particles: Advantages, Limitations, and Artifacts. Macromolecules 2006, 39, 2441–2444. [Google Scholar] [CrossRef]
- Hu, X.; Tong, Z.; Lyon, L.A. Multicompartment Core/Shell Microgels. J. Am. Chem. Soc. 2010, 132, 11470–11472. [Google Scholar] [CrossRef] [PubMed]
- Touve, M.A.; Figg, C.A.; Wright, D.B.; Park, C.; Cantlon, J.; Sumerlin, B.S.; Gianneschi, N.C. Polymerization-Induced Self-Assembly of Micelles Observed by Liquid Cell Transmission Electron Microscopy. ACS Cent. Sci. 2018, 4, 543–547. [Google Scholar] [CrossRef]
- Parent, L.R.; Bakalis, E.; Proetto, M.; Li, Y.; Park, C.; Zerbetto, F.; Gianneschi, N.C. Tackling the Challenges of Dynamic Experiments Using Liquid-Cell Transmission Electron Microscopy. Acc. Chem. Res. 2018, 51, 3–11. [Google Scholar] [CrossRef]
- Parent, L.R.; Bakalis, E.; Ramírez-Hernández, A.; Kammeyer, J.K.; Park, C.; de Pablo, J.; Zerbetto, F.; Patterson, J.P.; Gianneschi, N.C. Directly Observing Micelle Fusion and Growth in Solution by Liquid-Cell Transmission Electron Microscopy. J. Am. Chem. Soc. 2017, 139, 17140–17151. [Google Scholar] [CrossRef]
- Lansalot, M.; Rieger, J. Polymerization-Induced Self-Assembly. Macromol. Rapid Commun. 2019, 40, 1800885. [Google Scholar] [CrossRef] [Green Version]
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef]
- Li, W.S.J.; Ladmiral, V.; Takeshima, H.; Satoh, K.; Kamigaito, M.; Semsarilar, M.; Negrell, C.; Lacroix-Desmazes, P.; Caillol, S. Ferulic acid-based reactive core–shell latex by seeded emulsion polymerization. Polym. Chem. 2019, 10, 3116–3126. [Google Scholar] [CrossRef]
- Imai, H.; Shima, T.; Sutoh, K.; Walker, M.L.; Knight, P.J.; Kon, T.; Burgess, S.A. Direct observation shows superposition and large scale flexibility within cytoplasmic dynein motors moving along microtubules. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarff, C.A.; Fuller, M.J.G.; Thompson, R.F.; Iadaza, M.G. Variations on Negative Stain Electron Microscopy Methods: Tools for Tackling Challenging Systems. J. Vis. Exp. 2018, 57199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakovlev, S.; Libera, M. Dose-limited spectroscopic imaging of soft materials by low-loss EELS in the scanning transmission electron microscope. Micron 2008, 39, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, C.; Fuchs, G.; Plummer, C.J.G.; Stadelmann, P.A. The morphology of submicronsized core–shell latex particles: An electron microscopy study. Micron 2007, 38, 522–535. [Google Scholar] [CrossRef]
- Harris, J.R.; Roos, C.; Djalali, R.; Rheingans, O.; Maskos, M.; Schmidt, M. Application of the negative staining technique to both aqueous and organic solvent solutions of polymer particles. Micron 1999, 30, 289–298. [Google Scholar] [CrossRef]
- Ferguson, C.J.; Russell, G.T.; Gilbert, R.G. Synthesis of latices with polystyrene cores and poly(vinyl acetate) shells. 1. Use of polystyrene seeds. Polymer 2002, 43, 6371–6382. [Google Scholar] [CrossRef]
- Fairley, N.; Hoang, B.; Allen, C. Morphological Control of Poly(ethylene glycol)- block -poly(ε-caprolactone) Copolymer Aggregates in Aqueous Solution. Biomacromolecules 2008, 9, 2283–2291. [Google Scholar] [CrossRef]
- Geng, X.; Zhai, M.X.; Sun, T.; Meyers, G. Morphology Observation of Latex Particles with Scanning Transmission Electron Microscopy by a Hydroxyethyl Cellulose Embedding Combined with RuO4 Staining Method. Microsc. Microanal. 2013, 19, 319–326. [Google Scholar] [CrossRef]
- Trent, J.S. Ruthenium tetraoxide staining of polymers: New preparative methods for electron microscopy. Macromolecules 1984, 17, 2930–2931. [Google Scholar] [CrossRef]
- Antonietti, M.; Berton, B.; Göltner, C.; Hentze, H.-P. Synthesis of Mesoporous Silica with Large Pores and Bimodal Pore Size Distribution by Templating of Polymer Latices. Adv. Mater. 1998, 10, 154–159. [Google Scholar] [CrossRef]
- Pusch, J.; van Herk, A.M. Emulsion Polymerization of Transparent Core−Shell Latices with a Polydivinylbenzene Styrene and Vinyl Acetate. Macromolecules 2005, 38, 6909–6914. [Google Scholar] [CrossRef]
- Hanus, L.H.; Ploehn, H.J. Conversion of Intensity-Averaged Photon Correlation Spectroscopy Measurements to Number-Averaged Particle Size Distributions. 1. Theoretical Development. Langmuir 1999, 15, 3091–3100. [Google Scholar] [CrossRef]
- Renz, P.; Kokkinopoulou, M.; Landfester, K.; Lieberwirth, I. Imaging of Polymeric Nanoparticles: Hard Challenge for Soft Objects. Macromol. Chem. Phys. 2016, 217, 1879–1885. [Google Scholar] [CrossRef]
- Michen, B.; Geers, C.; Vanhecke, D.; Endes, C.; Rothen-Rutishauser, B.; Balog, S.; Petri-Fink, A. Avoiding drying-artifacts in transmission electron microscopy: Characterizing the size and colloidal state of nanoparticles. Sci. Rep. 2015, 5, 9793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackiewicz, M.; Stojek, Z.; Karbarz, M. Unusual swelling behavior of core-shell microgels built from polymers exhibiting lower critical solubility temperature. Eur. Polym. J. 2017, 95, 314–322. [Google Scholar] [CrossRef]
- Vanparijs, N.; Nuhn, L.; Paluck, S.J.; Kokkinopoulou, M.; Lieberwirth, I.; Maynard, H.D.; De Geest, B.G. Core/shell protein-reactive nanogels via a combination of RAFT polymerization and vinyl sulfone postmodification. Nanomedicine 2016, 11, 2631–2645. [Google Scholar] [CrossRef] [Green Version]
- Vladár, A.E.; Hodoroaba, V.-D. Characterization of nanoparticles by scanning electron microscopy. In Characterization of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 7–27. ISBN 978-0-12-814182-3. [Google Scholar]
- Ul-Hamid, A. A Beginners’ Guide to Scanning Electron Microscopy; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-98481-0. [Google Scholar]






| Microscopic Method | PHEA85–b–PS130 | PHEA23–b–PS130 |
|---|---|---|
| Dp, nm | ||
| TEM | 19.7 ± 3.0 | 36.4 ± 4.8 |
| TEM with RuO4-positive staining | 31.1 ± 3.8 | 41.8 ± 7.2 |
| STEM | 20.2 ± 1.7 | 34.8 ± 2.6 |
| STEM with RuO4-positive staining | 28.1 ± 2.2 | 42.6 ± 4.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tkachenko, V.; Vidal, L.; Josien, L.; Schmutz, M.; Poly, J.; Chemtob, A. Characterizing the Core-Shell Architecture of Block Copolymer Nanoparticles with Electron Microscopy: A Multi-Technique Approach. Polymers 2020, 12, 1656. https://doi.org/10.3390/polym12081656
Tkachenko V, Vidal L, Josien L, Schmutz M, Poly J, Chemtob A. Characterizing the Core-Shell Architecture of Block Copolymer Nanoparticles with Electron Microscopy: A Multi-Technique Approach. Polymers. 2020; 12(8):1656. https://doi.org/10.3390/polym12081656
Chicago/Turabian StyleTkachenko, Vitalii, Loïc Vidal, Ludovic Josien, Marc Schmutz, Julien Poly, and Abraham Chemtob. 2020. "Characterizing the Core-Shell Architecture of Block Copolymer Nanoparticles with Electron Microscopy: A Multi-Technique Approach" Polymers 12, no. 8: 1656. https://doi.org/10.3390/polym12081656
APA StyleTkachenko, V., Vidal, L., Josien, L., Schmutz, M., Poly, J., & Chemtob, A. (2020). Characterizing the Core-Shell Architecture of Block Copolymer Nanoparticles with Electron Microscopy: A Multi-Technique Approach. Polymers, 12(8), 1656. https://doi.org/10.3390/polym12081656