Modeling of Metalized Food Packaging Plastics Pyrolysis Kinetics Using an Independent Parallel Reactions Kinetic Model
Abstract
:1. Introduction
2. Experimental
2.1. Materials Selection and Analysis
2.2. Thermogravimetric Analysis
2.3. Chemical Analysis of the Formulated Chemical Compounds
2.4. Kinetic Models of MFPWs Pyrolysis
2.4.1. Independent Parallel Reactions Kinetic Model
2.4.2. Modified Independent Parallel Reactions Kinetic Model
3. Results and Discussions
3.1. Elemental and Proximate Analyses
3.2. Thermogravimetric Analysis
3.3. Chemical Analysis of the Synthesized Chemical Compounds Using FTIR and GC-MS
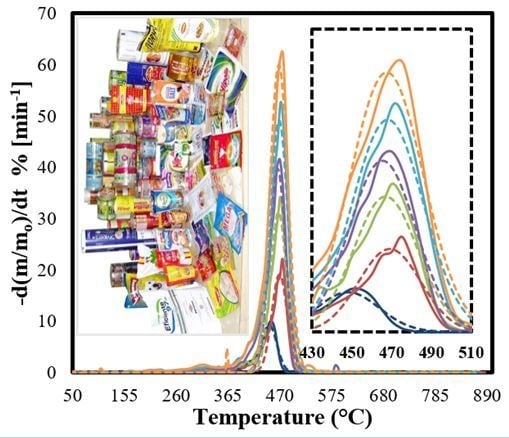
3.4. Kinetic Evaluation of the Pyrolysis of MFPWs
3.5. IPR Kinetic Model
3.6. Modified IPR Kinetic Model
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yin, S.; Rajarao, R.; Gong, B.; Wang, Y.; Kong, C.; Sahajwalla, V. Thermo-delamination of metallised composite plastic: An innovative approach to generate Aluminium from packaging plastic waste. J. Clean. Prod. 2019, 211, 321–329. [Google Scholar] [CrossRef]
- Struller, C.F.; Kelly, P.J.; Copeland, N.J. Aluminum oxide barrier coatings on polymer films for food packaging applications. Surf. Coat. Technol. 2014, 241, 130–137. [Google Scholar] [CrossRef]
- Mumladze, T.; Yousef, S.; Tatariants, M.; Kriukiene, R.; Makarevicius, V.; Lukošiute, S.I.; Bendikiene, R.; Denafas, G. Sustainable approach to recycling of multilayer flexible packaging using switchable hydrophilicity solvents. Green Chem. 2018, 20, 3604–3618. [Google Scholar] [CrossRef]
- Cervantes–Reyes, A.; Núñez–Pineda, A.; Barrera–Díaz, C.; Varela–Guerrero, V.; Martínez–Barrera, G.; Cuevas–Yañez, E. Solvent effect in the polyethylene recovery from multilayer postconsumer aseptic packaging. Waste Manag. 2015, 38, 61–64. [Google Scholar] [CrossRef]
- Xie, M.; Bai, W.; Bai, L.; Sun, X.; Lu, Q.; Yan, D.; Qiao, Q. Life cycle assessment of the recycling of Al–PE (a laminated foil made from polyethylene and aluminum foil) composite packaging waste. J. Clean. Prod. 2010, 112, 4430–4434. [Google Scholar] [CrossRef]
- Restuccia, D.; Spizzirri, U.G.; Parisi, O.I.; Cirillo, G.; Curcio, M.; Iemma, F.; Puoci, F.; Vinci, G.; Picci, N. New EU regulation aspects and global market of active and intelligent packaging for food industry applications. Food Control 2010, 21, 1425–1435. [Google Scholar] [CrossRef]
- Yousef, S.; Mumladze, T.; Tatariants, M.; Kriūkienė, R.; Makarevicius, V.; Bendikiene, R.; Denafas, G. Cleaner and profitable industrial technology for full recovery of metallic and non–metallic fraction of waste pharmaceutical blisters using switchable hydrophilicity solvents. J. Clean. Prod. 2018, 197, 379–392. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Horodytska, O.; Valdés, F.J.; Fullana, A. Plastic flexible films waste management—A state of art review. Waste Manag. 2018, 77, 413–425. [Google Scholar] [CrossRef]
- Möllnitz, S.; Khodier, K.; Pomberger, R.; Sarc, R. Grain size dependent distribution of different plastic types in coarse shredded mixed commercial and municipal waste. Waste Manag. 2020, 103, 388–398. [Google Scholar] [CrossRef]
- Chen, D.; Yin, L.; Wang, H.; He, P. Pyrolysis technologies for municipal solid waste: A review. Waste Manag. 2014, 34, 2466–2486. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Peng, Z.; Liu, Y.; Li, L.; Huang, Q.; Xie, M.; Wang, Q. Optimizing and developing a continuous separation system for the wet process separation of aluminum and polyethylene in aseptic composite packaging waste. Waste Manag. 2015, 35, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Meshram, A.; Singh, K.K. Recovery of valuable products from hazardous aluminum dross: A. review. Resour. Conserv. Recy. 2018, 130, 95–108. [Google Scholar] [CrossRef]
- Mahmood, A.A.; Hossain, R.; Sahajwalla, V. Microrecycling of the metal-polymer-laminated packaging materials via thermal disengagement technology. SN Appl. Sci. 2019, 1, 1106. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, A.A.; Hossain, R.; Bhattacharyya, B.; Sahajwalla, V. Recycling of polymer laminated aluminum packaging (PLAP) materials into carbonaceous metallic microparticles. J. Clean. Prod. 2020, 269, 122157. [Google Scholar] [CrossRef]
- Lu, C.H.; Chiang, K.Y. Gasification of non-recycled plastic packaging material containing aluminum: Hydrogen energy production and aluminum recovery. Int. J. Hydrogen Energy 2017, 44, 27532–27542. [Google Scholar] [CrossRef]
- Siddiqui, M.Z.; Park, Y.K.; Kang, Y.; Watanabe, A.; Kim, S.; Kim, Y.M. Effective use of aluminum-plastic laminate as a feedstock for catalytic pyrolysis over micro and mesoporous catalysts. J. Clean. Prod. 2019, 229, 1093–1101. [Google Scholar] [CrossRef]
- Quesada, L.; Pérez, A.; Godoy, V.; Peula, F.J.; Calero, M.; Blázquez, G. Optimization of the pyrolysis process of a plastic waste to obtain a liquid fuel using different mathematical models. Energy Convers. Manag. 2019, 118, 19–26. [Google Scholar] [CrossRef]
- Chhabra, V.; Shastri, Y.; Bhattacharya, S. Kinetics of Pyrolysis of Mixed Municipal Solid Waste—A Review. Procedia Environ. Sci. 2016, 35, 513–527. [Google Scholar] [CrossRef]
- Khoo, H.H. LCA of plastic waste recovery into recycled materials, energy and fuels in Singapore. Resour. Conserv. Recy. 2019, 145, 67–77. [Google Scholar] [CrossRef]
- Undri, A.; Rosi, L.; Frediani, M.; Frediani, P. Fuel from microwave assisted pyrolysis of waste multilayer packaging beverage. Fuel 2014, 133, 7–16. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Striūgas, N.; Zakarauskas, K.; Praspaliauskas, M.; Abdelnaby, M.A. Pyrolysis kinetic behavior and TG-FTIR-GC–MS analysis of metallised food packaging plastics. Fuel 2020, 282, 118737. [Google Scholar] [CrossRef]
- Veksha, A.; Yin, K.; Moo, J.G.S.; Oh, W.; Da Ahamed, A.; Chen, W.Q.; Piyarat, W.; Apostolos, G.; Grzegorz, L. Processing of flexible plastic packaging waste into pyrolysis oil and multi-walled carbon nanotubes for electrocatalytic oxygen reduction. J. Hazard. Mater. 2020, 121256. [Google Scholar] [CrossRef] [PubMed]
- Tuffi, R.; D’Abramo, S.; Cafiero, L.M.; Trinca, E.; Vecchio, C.S. Thermal behavior and pyrolytic degradation kinetics of polymeric mixtures from waste packaging plastics. Express Polym. Lett. 2018, 12, 82–99. [Google Scholar] [CrossRef]
- Das, P.; Tiwari, P. The effect of slow pyrolysis on the conversion of packaging waste plastics (PE and PP) into fuel. Waste Manag. 2018, 79, 615–624. [Google Scholar] [CrossRef]
- Hujuri, U.; Ghoshal, A.K.; Gumma, S. Modeling pyrolysis kinetics of plastic mixtures. Polym. Degrad. Stabil. 2008, 93, 1832–1837. [Google Scholar] [CrossRef]
- Zheng, Y.; Tao, L.; Yang, X.; Huang, Y.; Liu, C.; Zheng, Z. Study of the thermal behavior, kinetics, and product characterization of biomass and low-density polyethylene co-pyrolysis by thermogravimetric analysis and pyrolysis-GC/MS. J. Anal. Appl. Pyrol. 2018, 133, 185–197. [Google Scholar] [CrossRef]
- Kai, X.; Yang, T.; Shen, S.; & Li, R. TG-FTIR-MS study of synergistic effects during co-pyrolysis of corn stalk and high-density polyethylene (HDPE). Energ. Conver. Manag. 2019, 181, 202–213. [Google Scholar] [CrossRef]
- Yao, Z.; Yu, S.; Su, W.; Wu, W.; Tang, J.; Qi, W. Kinetic studies on the pyrolysis of plastic waste using a combination of model-fitting and model-free methods. Waste Manag. Res. 2020, 38, 77–85. [Google Scholar] [CrossRef]
- Sfakiotakis, S.; Vamvuka, D. Development of a modified independent parallel reactions kinetic model and comparison with the distributed activation energy model for the pyrolysis of a wide variety of biomass fuels. Bioresour. Technol. 2015, 197, 434–442. [Google Scholar] [CrossRef]
- Chhabra, V.; Bhattacharya, S.; Shastri, Y. Pyrolysis of mixed municipal solid waste: Characterisation, interaction effect and kinetic modelling using the thermogravimetric approach. Waste Manag. 2019, 90, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Quesada, L.; Calero, M.; Martín–Lara, M.A.; Pérez, A.; Blázquez, G. Characterization of fuel produced by pyrolysis of plastic film obtained of municipal solid waste. Energy 2019, 186. [Google Scholar] [CrossRef]
- Budsaereechai, S.; Hunt, A.J.; Ngernyen, Y. Catalytic pyrolysis of plastic waste for the production of liquid fuels for engines. RSC Adv. 2019, 9, 5844–5857. [Google Scholar] [CrossRef] [Green Version]
- Santos, K.G.; Lobato, F.S.; Lira, T.S.; Murata, V.V.; Barrozo, M.A.S. Sensitivity analysis applied to independent parallel reaction model for pyrolysis of bagasse. Chem. Eng. Res. Des. 2012, 90, 1989–1996. [Google Scholar] [CrossRef]
- Wang, B.; Xu, F.; Zong, P.; Zhang, J.; Tian, Y.; Qiao, Y. Effects of heating rate on fast pyrolysis behavior and product distribution of Jerusalem artichoke stalk by using TG-FTIR and Py-GC/MS. Renew. Energy 2019, 132, 486–496. [Google Scholar] [CrossRef]
- Zong, P.; Jiang, Y.; Tian, Y.; Li, J.; Yuan, M.; Ji, Y.; Chen, M.; Li, D.; Qiao, Y. Pyrolysis behavior and product distributions of biomass six group components: Starch, cellulose, hemicellulose, lignin, protein and oil. Energy Conver. Manag. 2020, 216, 112777. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, B.; Zong, P.; Tian, Y.; Xu, F.; Li, D.; Li, F.; Tian, Y. Thermal behavior, kinetics and fast pyrolysis characteristics of palm oil: Analytical TG-FTIR and Py-GC/MS study. Energy Conver. Manag. 2019, 199, 111964. [Google Scholar] [CrossRef]
- Shen, Y. A review on hydrothermal carbonization of biomass and plastic wastes to energy products. Biomass Bioenergy 2020, 134, 105479. [Google Scholar] [CrossRef]
- Islam, M.N.; Beg, M.R.A. Fixed Bed Pyrolysis of Waste Plastic for Alternative Fuel Production. J. Energy Environ. 2004, 3, 69–80. [Google Scholar]
- Yousef, S.; Eimontas, J.; Striūgas, N.; Tatariants, M.; Abdelnaby, M.A.; Tuckute, S.; Kliucininkas, L. A sustainable bioenergy conversion strategy for textile waste with self-catalysts using mini-pyrolysis plant. Energy Conver. Manag. 2019, 196, 688–704. [Google Scholar] [CrossRef]
- Rago, Y.P.; Collard, F.X.; Görgens, J.F.; Surroop, D.; Mohee, R. Torrefaction of biomass and plastic from municipal solid waste streams and their blends: Evaluation of interactive effects. Fuel 2020, 277, 118089. [Google Scholar] [CrossRef]
- Ahamed, A.; Veksha, A.; Yin, K.; Weerachanchai, P.; Giannis, A.; Lisak, G. Environmental impact assessment of converting flexible packaging plastic waste to pyrolysis oil and multi-walled carbon nanotubes. J. Hazard. Mater. 2020, 390, 121449. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, H.; Kumari, U.; Biswas, S.; Mishra, A.; Meikap, B.C. A synergistic study of reaction kinetics and heat transfer with multi-component modelling approach for the pyrolysis of biomass waste. Energy 2020, 117933. [Google Scholar] [CrossRef]
- Ma, C.; Yan, Q.; Yu, J.; Chen, T.; Wang, D.; Liu, S.; Bikane, K.; Sun, L. The behavior of heteroatom compounds during the pyrolysis of waste computer casing plastic under various heating conditions. J. Clean. Prod. 2019, 219, 461–470. [Google Scholar] [CrossRef]
- Ma, W.; Rajput, G.; Pan, M.; Lin, F.; Zhong, L.; Chen, G. Pyrolysis of typical MSW components by Py-GC/MS and TG-FTIR. Fuel 2019, 251, 693–708. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P. Thermal degradation of waste plastics under non-sweeping atmosphere: Part 2: Effect of process temperature on product characteristics and their future applications. J. Environ. Manag. 2020, 261, 110112. [Google Scholar] [CrossRef]
- Schwartz, N.R.; Paulsen, A.D.; Blaise, M.J.; Wagner, A.L.; Yelvington, P.E. Analysis of emissions from combusting pyrolysis products. Fuel 2020, 274, 117863. [Google Scholar] [CrossRef]
- Kasperski, J.; Grabowska, B. The effect of plastic film transmittance on heat transfer in a multilayer insulation structure of rectangular air cells for frozen food wrapping. Int. J. Refrig. 2018, 92, 94–105. [Google Scholar] [CrossRef]
- Navarro, M.V.; López, J.M.; Veses, A.; Callén, M.S.; García, T. Kinetic study for the co-pyrolysis of lignocellulosic biomass and plastics using the distributed activation energy model. Energy 2018, 165, 731–742. [Google Scholar] [CrossRef]
- Wu, X.; Wu, Y.; Wu, K.; Chen, Y.; Hu, H.; Yang, M. Study on pyrolytic kinetics and behavior: The co-pyrolysis of microalgae and polypropylene. Bioresour. Technol. 2015, 192, 522–528. [Google Scholar] [CrossRef]
- Aboulkas, A.; El harfi, K.; El Bouadili, A. Thermal degradation behaviors of polyethylene and polypropylene. Part I: Pyrolysis kinetics and mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Till, Z.; Varga, T.; Sója, J.; Miskolczi, N.; Chován, T. Kinetic identification of plastic waste pyrolysis on zeolite-based catalysts. Energy Convers. Manag. 2018, 173, 320–330. [Google Scholar] [CrossRef]
- Patnaik, S.; Panda, A.K.; Kumar, S. Thermal degradation of corn starch based biodegradable plastic plates and determination of kinetic parameters by isoconversional methods using thermogravimetric analyzer. J. Energy Inst. 2020. [Google Scholar] [CrossRef]




| Equation No. | Parameters | Definition |
|---|---|---|
| 2 | mo, mt, mf | The initial, instantaneous, and final mass of the sample, respectively |
| 3 | , , Ei, ni, t, T, R | The conversion, pre-exponential factor, activation energy, apparent reaction order of each subcomponent i, time, temperature, and the universal gas constant, respectively. |
| 4 | Ci | The mass fraction of each of the three subcomponents |
| 5 | dm/dt | The rate of mass loss |
| 6 | Derivative Thermogravimetry (DTG) objective function | |
| 7 | Z, N | The number of data points and the number of parameters employed in the model, respectively. |
| Sample Code | Elemental Analysis (wt %) | Proximate Analysis (wt %) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O | Moisture | Volatile Matter | Fixed Carbon | Ash | |
| MFPW1 | 82.64 | 14.58 | 0.74 | 0.01 | 3.84 | 0.23 | 93.87 | 4.98 | 1.07 |
| MFPW2 | 80.75 | 13.92 | 0.48 | 0.02 | 4.02 | 0.35 | 95.03 | 3.67 | 1.22 |
| MFPW3 | 81.28 | 14.81 | 0.52 | 0.01 | 3.86 | 0.16 | 72.14 | 22.54 | 5.28 |
| Average | 81.56 | 14.44 | 0.58 | 0.01 | 3.91 | 0.25 | 87.01 | 10.40 | 2.52 |
| Pyrolysis Parameters | Heating Rate (°C min−1) | |||||
|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | 25 | 30 | |
| Chips sample | ||||||
| Ti (°C) | 321.85 | 408.94 | 416.13 | 416.7 | 418.7 | 418.8 |
| Tm (°C) | 448.4 | 458.2 | 465.88 | 466 | 469.4 | 470.4 |
| Tf (°C) | 464 | 494 | 508 | 512.37 | 517.17 | 564.003 |
| Rmax (%/min) | 13.8 | 30.47 | 42.42 | 53.44 | 72.27 | 91.19 |
| Di (% min−1 °C−3) | 3.4 × 10−6 | 5.69 × 10−6 | 6.97 × 10−6 | 8.00 × 10−6 | 1.00 × 10−5 | 1.25 × 10−5 |
| 28.2 | 28.586 | 31.4 | 34.4 | 36.6 | 37.1 | |
| Mf (%) | 0.073 | 1.614 | 2.183 | 2.92 | 2.4 | 0.01 |
| Chocolate sample | ||||||
| Ti (°C) | 306.1 | 306.94 | 309.4 | 315.95 | 338 | 340.49 |
| Tm (°C) | 450.526 | 466.74 | 471.12 | 471.02 | 472.4 | 465 |
| Tf (°C) | 555.316 | 557 | 566.31 | 573.1 | 580.91 | 591 |
| Rmax (%/min) | 12.6 | 21.77 | 39 | 79.05 | 80.4 | 81.78 |
| Di (% min−1 °C−3) | 3.45 × 10−6 | 4.34 × 10−6 | 9.91 × 10−6 | 3.32 × 10−5 | 2.65 × 10−5 | 2.72 × 10−5 |
| 26.457 | 35 | 27 | 16 | 19 | 19 | |
| Mf (%) | 7.9 | 3.95 | 0.31 | 3.1 | 3.2 | 3.3 |
| Coffee sample | ||||||
| Ti (°C) | 372.72 | 380.4 | 381.311 | 384 | 392 | 394.8 |
| Tm (°C) | 464.5 | 475 | 478.63 | 485.8 | 486 | 486 |
| Tf (°C) | 484.8 | 495.6 | 502.3 | 512.81 | 527.33 | 585.46 |
| Rmax (%/min) | 6.5734 | 15.98 | 19.2 | 26.55 | 31.3792 | 39.2365 |
| Di (% min−1 °C−3) | 1.19 × 10−6 | 2.77 × 10−6 | 2.59 × 10−6 | 3.08 × 10−6 | 3.23 × 10−6 | 3.95 × 10−6 |
| 31.8 | 31.95 | 40.6 | 46.14 | 50.9707 | 51.815 | |
| Mf (%) | 34.688 | 30.449 | 29.604 | 29.8491 | 33.2312 | 30.4352 |
| The mixed sample | ||||||
| Ti (°C) | 378 | 384.67 | 388 | 391 | 384.19 | 385.19 |
| Tm (°C) | 449.64 | 465 | 470.05 | 470.13 | 471.7 | 476 |
| Tf (°C) | 515 | 521 | 537 | 556 | 581 | 593 |
| Rmax (%/min) | 9.9123 | 22.1084 | 34.3 | 41.65 | 52.8447 | 62.04 |
| Di (% min−1 °C−3) | 1.62 × 10−6 | 3.39 × 10−6 | 5.141 × 10−6 | 5.96 × 10−6 | 7.33 × 10−6 | 8.25 × 10−6 |
| 36 | 36.5 | 36.6 | 38 | 39.8 | 41 | |
| Mf (%) | 15.2 | 14.8 | 11.29 | 14.92 | 11.06 | 13.07 |
| Parameter | Chips | Chocolate | Coffee | Mixture |
|---|---|---|---|---|
| E1 | 145.1549 | 133.9133 | 143.0858 | 163.3006 |
| A1 (at 30 °C/min) | 2.86 × 1013 | 1.19 × 1012 | 3.92 × 1013 | 6.44 × 1014 |
| c1 | 0.253553 | 0.297861 | 0.301881 | 0.388108 |
| E2 | 261.7056 | 241.4376 | 257.975 | 294.4211 |
| A2 (at 30 °C/min) | 2.56 × 1016 | 1.06 × 1015 | 3.51 × 1016 | 5.77 × 1017 |
| c2 | 0.554647 | 0.402139 | 0.339819 | 0.411892 |
| MIPR deviation (%) | 3.122 | 3.373 | 2.18 | 2.35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousef, S.; Eimontas, J.; Striūgas, N.; Abdelnaby, M.A. Modeling of Metalized Food Packaging Plastics Pyrolysis Kinetics Using an Independent Parallel Reactions Kinetic Model. Polymers 2020, 12, 1763. https://doi.org/10.3390/polym12081763
Yousef S, Eimontas J, Striūgas N, Abdelnaby MA. Modeling of Metalized Food Packaging Plastics Pyrolysis Kinetics Using an Independent Parallel Reactions Kinetic Model. Polymers. 2020; 12(8):1763. https://doi.org/10.3390/polym12081763
Chicago/Turabian StyleYousef, Samy, Justas Eimontas, Nerijus Striūgas, and Mohammed Ali Abdelnaby. 2020. "Modeling of Metalized Food Packaging Plastics Pyrolysis Kinetics Using an Independent Parallel Reactions Kinetic Model" Polymers 12, no. 8: 1763. https://doi.org/10.3390/polym12081763
APA StyleYousef, S., Eimontas, J., Striūgas, N., & Abdelnaby, M. A. (2020). Modeling of Metalized Food Packaging Plastics Pyrolysis Kinetics Using an Independent Parallel Reactions Kinetic Model. Polymers, 12(8), 1763. https://doi.org/10.3390/polym12081763