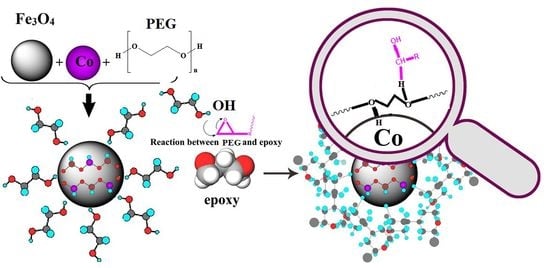
Bulk-Surface Modification of Nanoparticles for Developing Highly-Crosslinked Polymer Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of MNPs
2.2. Epoxy Nanocomposite Preparation
2.3. Characterization of Nanoparticles and Nanocomposites
3. Results and Discussion
3.1. Analysis of the Bulk and Surface of MNPs
3.2. Analysis of Cure Reaction
3.3. Glass Transition Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Isoconversional Kinetic Methods
Appendix A.1. Friedman Model

Appendix A.2. KAS Method

Appendix B. Selection of Curing Reaction Model
Friedman Model

Appendix C. Determination of Degree of Reaction


References
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Rong, M.; Zhang, M.; Ruan, W. Surface modification of nanoscale fillers for improving properties of polymer nanocomposites: A review. Mater. Sci. Technol. 2006, 22, 787–796. [Google Scholar] [CrossRef]
- Nonahal, M.; Saeb, M.R.; Hassan Jafari, S.; Rastin, H.; Khonakdar, H.A.; Najafi, F.; Simon, F. Design, preparation, and characterization of fast cure epoxy/amine-functionalized graphene oxide nanocomposites. Polym. Compos. 2018, 39, e2016–e2027. [Google Scholar] [CrossRef]
- Saeb, M.R.; Bakhshandeh, E.; Khonakdar, H.A.; Mäder, E.; Scheffler, C.; Heinrich, G. Cure kinetics of epoxy nanocomposites affected by MWCNTs functionalization: A review. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Nonahal, M.; Rastin, H.; Saeb, M.R.; Sari, M.G.; Moghadam, M.H.; Zarrintaj, P.; Ramezanzadeh, B. Epoxy/PAMAM dendrimer-modified graphene oxide nanocomposite coatings: Nonisothermal cure kinetics study. Progre. Org. Coat. 2018, 114, 233–243. [Google Scholar] [CrossRef]
- Sari, M.G.; Saeb, M.R.; Shabanian, M.; Khaleghi, M.; Vahabi, H.; Vagner, C.; Zarrintaj, P.; Khalili, R.; Paran, S.M.R.; Ramezanzadeh, B.; et al. Epoxy/starch-modified nano-zinc oxide transparent nanocomposite coatings: A showcase of superior curing behavior. Prog. Org. Coat. 2018, 115, 143–150. [Google Scholar] [CrossRef]
- Karami, Z.; Jazani, O.M.; Navarchian, A.H.; Karrabi, M.; Vahabi, H.; Saeb, M.R. Well-cured silicone/halloysite nanotubes nanocomposite coatings. Prog. Org. Coat. 2019, 129, 357–365. [Google Scholar] [CrossRef]
- Karami, Z.; Jazani, O.M.; Navarchian, A.H.; Saeb, M.R. State of cure in silicone/clay nanocomposite coatings: The puzzle and the solution. Prog. Org. Coat. 2018, 125, 222–233. [Google Scholar] [CrossRef]
- Akbari, V.; Najafi, F.; Vahabi, H.; Jouyandeh, M.; Badawi, M.; Morisset, S.; Ganjali, M.R.; Saeb, M.R. Surface chemistry of halloysite nanotubes controls the curability of low filled epoxy nanocomposites. Prog. Org. Coat. 2019, 135, 555–564. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Tang, R.P.-L.; Wong, K.M.-C.; Cheung, K.-K. Synthesis, luminescence, electrochemistry, and ion-binding studies of platinum (II) terpyridyl acetylide complexes. Organometallics 2001, 20, 4476–4482. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, K.; Zeng, C. Use of electrochemistry in the synthesis of heterocyclic structures. Chem. Rev. 2017, 118, 4485–4540. [Google Scholar] [CrossRef] [PubMed]
- Alsheyab, M.; Jiang, J.-Q.; Stanford, C. On-line production of ferrate with an electrochemical method and its potential application for wastewater treatment–A review. J. Environ. Manag. 2009, 90, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Scholz, R.; Nielsch, K.; Gösele, U. A template-based electrochemical method for the synthesis of multisegmented metallic nanotubes. Angew. Chem. Int. Ed. 2005, 44, 6050–6054. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh, M. One-step cathodic electrosynthesis of surface capped Fe3O4 ultra-fine nanoparticles from ethanol medium without using coating agent. Mater. Lett. 2018, 211, 225–229. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Karimzadeh, I. One-pot electro-synthesis and characterization of chitosan capped superparamagnetic Iron oxide nanoparticles (SPIONs) from ethanol media. Curr. Nanosci. 2018, 14, 42–49. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Karami, Z.; Ali, J.A.; Karimzadeh, I.; Aghazadeh, M.; Laoutid, F.; Vahabi, H.; Saeb, M.R.; Ganjali, M.R.; Dubois, P. Curing epoxy with polyethylene glycol (PEG) surface-functionalized NixFe3-xO4magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105250. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Karimzadeh, I.; Ganjali, M.R. Electrochemical evaluation of the performance of cathodically grown ultra-fine magnetite nanoparticles as electrode material for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2017, 28, 13532–13539. [Google Scholar] [CrossRef]
- Karimzadeh, I.; Aghazadeh, M.; Doroudi, T.; Reza Ganjali, M.; Hossein Kolivand, P. Effective Preparation, Characterization and In Situ Surface Coating of Superparamagnetic Fe3O4 Nanoparticles with Polyethyleneimine Through Cathodic Electrochemical Deposition (CED). Curr. Nanosci. 2017, 13, 167–174. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Ganjali, M.R. One-pot electrochemical synthesis and assessment of super-capacitive and super-paramagnetic performances of Co2+ doped Fe3O4 ultra-fine particles. J. Mater. Sci. Mater. Electron. 2018, 29, 2291–2300. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Karimzadeh, I.; Ganjali, M.R. PVP capped Mn2+ doped Fe3O4 nanoparticles: A novel preparation method, surface engineering and characterization. Mater. Lett. 2018, 228, 137–140. [Google Scholar] [CrossRef]
- Aghazadeh, M. Electrochemical Synthesis of Dextran-and Polyethyleneimine-Coated Superparamagnetic Iron Oxide Nanoparticles and Investigation of their Physico-Chemical Characters. Anal. Bioanal. Electrochem. 2019, 11, 362–372. [Google Scholar]
- Aghazadeh, M.; Karimzadeh, I.; Ganjali, M.R.; Behzad, A. Mn2+-doped Fe3O4 nanoparticles: A novel preparation method, structural, magnetic and electrochemical characterizations. J. Mater. Sci. Mater. Electron. 2017, 28, 18121–18129. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ali, J.A.; Aghazadeh, M.; Formela, K.; Saeb, M.R.; Ranjbar, Z.; Ganjali, M.R. Curing epoxy with electrochemically synthesized ZnxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105246. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Hamad, S.M.; Karimzadeh, I.; Aghazadeh, M.; Karami, Z.; Akbari, V.; Shammiry, F.; Formela, K.; Saeb, M.R.; Ranjbar, Z.; et al. Unconditionally blue: Curing epoxy with polyethylene glycol (PEG) surface-functionalized ZnxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 137, 105285. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Aghazadeh, M.; Stadler, F.J.; Saeb, M.R. Curing epoxy with electrochemically synthesized NixFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105198. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Zarrintaj, P.; Ganjali, M.R.; Ali, J.A.; Karimzadeh, I.; Aghazadeh, M.; Ghaffari, M.; Saeb, M.R. Curing epoxy with electrochemically synthesized GdxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105245. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Akbari, V.; Karami, Z.; Aghazadeh, M.; Zarrintaj, P.; Saeb, M.R. Curing epoxy with polyethylene glycol (PEG) surface-functionalized GdxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 137, 105283. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Aghazadeh, M.; Stadler, F.J.; Saeb, M.R. Curing epoxy with electrochemically synthesized CoxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 137, 105252. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Aghazadeh, M.; Karimzadeh, I.; Formela, K.; Colom, X.; Cañavate, J.; Saeb, M.R. Curing epoxy with ethylenediaminetetraacetic acid (EDTA) surface-functionalized CoxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105248. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Hadavand, B.S.; Aghazadeh, M.; Akbari, V.; Shammiry, F.; Saeb, M.R. Curing epoxy with polyvinyl chloride (PVC) surface-functionalized CoxFe3-xO4 nanoparticles. Prog. Org. Coat. 2019, 137, 105364. [Google Scholar] [CrossRef]
- Aghazadeh, M. Cathodic Electrochemical Deposition of Nanostructured Metal Oxides/Hydroxides and their Composites for Supercapacitor Applications: A Review; Anal. Bioanal. Electrochem. 2019, 11, 211–266. [Google Scholar]
- Aghazadeh, M.; Ganjali, M.R. One-step electro-synthesis of Ni2+ doped magnetite nanoparticles and study of their supercapacitive and superparamagnetic behaviors. J. Mater. Sci. Mater. Electron. 2018, 29, 4981–4991. [Google Scholar] [CrossRef]
- Aghazadeh, M. Zn-doped magnetite nanoparticles: Development of novel preparation method and evaluation of magnetic and electrochemical capacitance performances. J. Mater. Sci. Mater. Electron. 2017, 28, 18755–18764. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Karimzadeh, I.; Ganjali, M.R.; Maragheh, M.G. Electrochemical fabrication of praseodymium cations doped iron oxide nanoparticles with enhanced charge storage and magnetic capabilities. J. Mater. Sci. Mater. Electron. 2018, 29, 5163–5172. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Zhang, Q.; Wang, Q.; Xing, S. One-pot Synthesis of Cobalt-doped Fe3O4 with Enhanced Catalytic Activity for Ozonation of 2, 4-Dichlorophenoxyacetic Acid (2, 4-D) in Aqueous Solution. Chem. Lett. 2015, 44, 785–787. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Moini Jazani, O.; Navarchian, A.H.; Saeb, M.R. High-performance epoxy-based adhesives reinforced with alumina and silica for carbon fiber composite/steel bonded joints. J. Reinf. Plast. Compo. 2016, 35, 1685–1695. [Google Scholar] [CrossRef]
- Aliakbari, M.; Jazani, O.M.; Sohrabian, M.; Jouyandeh, M.; Saeb, M.R. Multi-nationality epoxy adhesives on trial for future nanocomposite developments. Prog. Org. Coat. 2019, 133, 376–386. [Google Scholar] [CrossRef]
- Vahabi, H.; Jouyandeh, M.; Cochez, M.; Khalili, R.; Vagner, C.; Ferriol, M.; Movahedifar, E.; Ramezanzadeh, B.; Rostami, M.; Ranjbar, Z.; et al. Short-lasting fire in partially and completely cured epoxy coatings containing expandable graphite and halloysite nanotube additives. Prog. Org. Coat. 2018, 123, 160–167. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Jazani, O.M.; Navarchian, A.H.; Saeb, M.R. Epoxy Coatings Physically Cured with Hydroxyl-contained Silica Nanospheres and Halloysite nanotubes. Prog. Color Color. Coat. 2018, 11, 199–207. [Google Scholar]
- Jouyandeh, M.; Ganjali, M.R.; Ali, J.A.; Aghazadeh, M.; Paran, S.M.R.; Naderi, G.; Saeb, M.R.; Thomas, S. Curing epoxy with polyvinylpyrrolidone (PVP) surface-functionalized ZnxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105227. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Yarahmadi, E.; Didehban, K.; Ghiyasi, S.; Paran, S.M.R.; Puglia, D.; Ali, J.A.; Jannesari, A.; Saeb, M.R.; Ranjbar, Z.; et al. Cure kinetics of epoxy/graphene oxide (GO) nanocomposites: Effect of starch functionalization of GO nanosheets. Prog. Org. Coat. 2019, 136, 105217. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol. Rapid Commun. 2006, 27, 1515–1532. [Google Scholar] [CrossRef]
- Ratna, D. Handbook of Thermoset Resins; Ismithers: Shawbury, UK, 2009. [Google Scholar]
- Paran, S.M.R.; Vahabi, H.; Jouyandeh, M.; Ducos, F.; Formela, K.; Saeb, M.R. Thermal decomposition kinetics of dynamically vulcanized polyamide 6–acrylonitrile butadiene rubber–halloysite nanotube nanocomposites. J. Appl. Polym. Sci. 2019, 136, 47483. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Paran, S.M.R.; Jannesari, A.; Puglia, D.; Saeb, M.R. Protocol for nonisothermal cure analysis of thermoset composites. Prog. Org. Coat. 2019, 131, 333–339. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ali, J.A.; Akbari, V.; Aghazadeh, M.; Paran, S.M.R.; Naderi, G.; Saeb, M.R.; Ranjbar, Z.; Ganjali, M.R. Curing epoxy with polyvinylpyrrolidone (PVP) surface-functionalized MnxFe3-xO4 magnetic nanoparticles. Prog. Org. Coat. 2019, 136, 105247. [Google Scholar] [CrossRef]
- Fu, Z.; Yang, B.; Zhang, Y.; Zhang, N.; Yang, Z. Dopant segregation and CO adsorption on doped Fe3O4 (1 1 1) surfaces: A first-principle study. J. Catal. 2018, 364, 291–296. [Google Scholar] [CrossRef]
- Saeb, M.R.; Rastin, H.; Nonahal, M.; Ghaffari, M.; Jannesari, A.; Formela, K. Cure kinetics of epoxy/MWCNTs nanocomposites: Nonisothermal calorimetric and rheokinetic techniques. J. Appl. Polym. Sci. 2017, 134, 45221. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Paran, S.M.R.; Shabanian, M.; Ghiyasi, S.; Vahabi, H.; Badawi, M.; Formela, K.; Puglia, D.; Saeb, M.R. Curing behavior of epoxy/Fe3O4 nanocomposites: A comparison between the effects of bare Fe3O4, Fe3O4/SiO2/chitosan and Fe3O4/SiO2/chitosan/imide/phenylalanine-modified nanofillers. Prog. Org. Coat. 2018, 123, 10–19. [Google Scholar] [CrossRef]
- Yarahmadi, E.; Didehban, K.; Sari, M.G.; Saeb, M.R.; Shabanian, M.; Aryanasab, F.; Zarrintaj, P.; Paran, S.M.R.; Mozafari, M.; Rallini, M. Development and curing potential of epoxy/starch-functionalized graphene oxide nanocomposite coatings. Prog. Org. Coat. 2018, 119, 194–202. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Karami, Z.; Hamad, S.M.; Ganjali, M.R.; Akbari, V.; Vahabi, H.; Kim, S.-J.; Zarrintaj, P.; Saeb, M.R. Nonisothermal cure kinetics of epoxy/ZnxFe3-xO4 nanocomposites. Prog. Org. Coat. 2019, 136, 105290. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Paran, S.M.R.; Khadem, S.S.M.; Ganjali, M.R.; Akbari, V.; Vahabi, H.; Saeb, M.R. Nonisothermal cure kinetics of epoxy/MnxFe3-xO4 nanocomposites. Prog. Org. Coat. 2020, 140, 105505. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Akbari, V.; Jouyandeh, M.; Paran, S.M.R.; Ganjali, M.R.; Abdollahi, H.; Vahabi, H.; Ahmadi, Z.; Formela, K.; Esmaeili, A.; Mohaddespour, A. Effect of Surface Treatment of Halloysite Nanotubes (HNTs) on the Kinetics of Epoxy Resin Cure with Amines. Polymers 2020, 12, 930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidi, F.; Jouyandeh, M.; Akbari, V.; Paran, S.M.R.; Livi, S.; Ducos, F.; Vahabi, H.; Ganjali, M.R.; Saeb, M.R. Super-crosslinked ionic liquid-intercalated montmorillonite/epoxy nanocomposites: Cure kinetics, viscoelastic behavior and thermal degradation mechanism. Polym. Eng. Sci. 2020, 123, pen–25441. [Google Scholar] [CrossRef]
- Gotor, F.J.; Criado, J.M.; Malek, J.; Koga, N. Kinetic analysis of solid-state reactions: The universality of master plots for analyzing isothermal and nonisothermal experiments. J. Phys. Chem. A 2000, 104, 10777–10782. [Google Scholar] [CrossRef]
- Criado, J.; Malek, J.; Ortega, A. Applicability of the master plots in kinetic analysis of non-isothermal data. Thermochim. Acta 1989, 147, 377–385. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Ganjali, M.R.; Seidi, F.; Xiao, H.; Saeb, M.R. Nonisothermal Cure Kinetics of Epoxy/Polyvinylpyrrolidone Functionalized Superparamagnetic Nano-Fe3O4 Composites: Effect of Zn and Mn Doping. J. Compos. Sci. 2020, 4, 55. [Google Scholar] [CrossRef]
- Tikhani, F.; Moghari, S.; Jouyandeh, M.; Laoutid, F.; Vahabi, H.; Saeb, M.R.; Dubois, P. Curing Kinetics and Thermal Stability of Epoxy Composites Containing Newly Obtained Nano-Scale Aluminum Hypophosphite (AlPO2). Polymers 2020, 12, 644. [Google Scholar] [CrossRef] [Green Version]
 shows Good cure state, while
shows Good cure state, while  denotes Poor cure state.
denotes Poor cure state.
 shows Good cure state, while
shows Good cure state, while  denotes Poor cure state.
denotes Poor cure state.











| Designation | β (°C·min−1) | Tonset (°C) | Tendset (°C) | Tp (°C) | ΔT (°C) | ΔH∞ (J/g) | ΔT* | ΔH* | Cure Index | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| EP | 5 | 25.82 | 160.63 | 90.223 | 134.81 | 361.04 | n.a. | n.a. | n.a. | n.a. |
| 10 | 32.46 | 186.62 | 101.02 | 154.17 | 350.64 | n.a. | n.a. | n.a. | n.a. | |
| 15 | 34.64 | 202.64 | 109.94 | 168 | 314.54 | n.a. | n.a. | n.a. | n.a. | |
| 20 | 39.93 | 209.27 | 118.03 | 169.34 | 336.89 | n.a. | n.a. | n.a. | n.a. | |
| EP/PEG-MNPs | 5 | 29.61 | 157.45 | 90.173 | 127.84 | 308.13 | 0.95 | 0.85 | 0.81 | Poor |
| 10 | 42.66 | 186 | 100.88 | 143.34 | 368.31 | 0.93 | 1.05 | 0.98 | Excellent | |
| 15 | 47.43 | 197.45 | 109.12 | 150.03 | 384.11 | 0.89 | 1.22 | 1.09 | Excellent | |
| 20 | 57.55 | 199.92 | 115.88 | 142.36 | 346.27 | 0.84 | 1.03 | 0.86 | Excellent | |
| EP/Co-PEG- MNPs | 5 | 29.12 | 160.86 | 90.677 | 131.74 | 446.35 | 0.98 | 1.24 | 1.21 | Excellent |
| 10 | 33.91 | 170.41 | 100.49 | 136.50 | 396.33 | 0.89 | 1.13 | 1.00 | Excellent | |
| 15 | 41.39 | 176.38 | 107.98 | 135.00 | 365.32 | 0.80 | 1.16 | 0.93 | Excellent | |
| 20 | 47.56 | 186.56 | 113.62 | 139.00 | 389.25 | 0.82 | 1.16 | 0.95 | Excellent |
| Designation | Heating Rate (°C·min−1) | αp∞ | αm | αp |
|---|---|---|---|---|
| EP | 5 | 0.501 | 0.099 | 0.517 |
| 10 | 0.403 | 0.127 | 0.505 | |
| 15 | 0.413 | 0.228 | 0.484 | |
| 20 | 0.451 | 0.208 | 0.501 | |
| EP/PEG-MNPs | 5 | 0.505 | 0.126 | 0.527 |
| 10 | 0.333 | 0.113 | 0.485 | |
| 15 | 0.345 | 0.096 | 0.470 | |
| 20 | 0.341 | 0.113 | 0.467 | |
| EP/Co-PEG-MNPs | 5 | 0.484 | 0.113 | 0.508 |
| 10 | 0.550 | 0.076 | 0.495 | |
| 15 | 0.567 | 0.233 | 0.505 | |
| 20 | 0.510 | 0.249 | 0.488 |
| Friedman | ||||||||
| Designation | Heating Rate (°C·min−1) | Ēα (kJ/mol) | ln(A) (1/s) | Mean (1/s) | m | Mean | n | Mean |
| EP | 5 | 51.83 | 16.24 | 16.29 | 0.381 | 0.408 | 1.509 | 1.534 |
| 10 | 16.30 | 0.421 | 1.545 | |||||
| 15 | 16.28 | 0.383 | 1.539 | |||||
| 20 | 16.32 | 0.447 | 1.543 | |||||
| EP/PEG-MNPs | 5 | 54.30 | 16.93 | 17.14 | 0.256 | 0.374 | 1.499 | 1.630 |
| 10 | 17.10 | 0.335 | 1.637 | |||||
| 15 | 17.13 | 0.379 | 1.664 | |||||
| 20 | 17.39 | 0.528 | 1.719 | |||||
| EP/Co- PEG-MNPs | 5 | 65.86 | 21.01 | 21.05 | 0.354 | 0.380 | 1.661 | 1.662 |
| 10 | 21.02 | 0.341 | 1.648 | |||||
| 15 | 21.03 | 0.360 | 1.652 | |||||
| 20 | 21.13 | 0.464 | 1.687 | |||||
| KAS | ||||||||
| Designation | Heating Rate (°C·min−1) | Ēα (kJ/mol) | ln(A) (1/s) | Mean (1/s) | m | Mean | n | Mean |
| EP | 5 | 49.82 | 15.58 | 15.65 | 0.405 | 0.431 | 1.490 | 1.515 |
| 10 | 15.67 | 0.444 | 1.526 | |||||
| 15 | 15.65 | 0.405 | 1.519 | |||||
| 20 | 15.71 | 0.469 | 1.524 | |||||
| EP/PEG-MNPs | 5 | 50.37 | 15.63 | 15.89 | 0.303 | 0.416 | 1.459 | 1.589 |
| 10 | 15.85 | 0.377 | 1.594 | |||||
| 15 | 15.90 | 0.420 | 1.622 | |||||
| 20 | 16.18 | 0.564 | 1.679 | |||||
| EP/Co- PEG-MNPs | 5 | 61.82 | 19.68 | 19.76 | 0.397 | 0.421 | 1.626 | 1.627 |
| 10 | 19.73 | 0.384 | 1.612 | |||||
| 15 | 19.76 | 0.401 | 1.617 | |||||
| 20 | 19.87 | 0.502 | 1.653 | |||||
| Sample | Tg (°C) |
|---|---|
| EP | 101.1 |
| EP/PEG-MNPs | 102.6 |
| EP/Co-PEG-MNPs | 105.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jouyandeh, M.; Ganjali, M.R.; Aghazadeh, M.; Habibzadeh, S.; Formela, K.; Saeb, M.R. Bulk-Surface Modification of Nanoparticles for Developing Highly-Crosslinked Polymer Nanocomposites. Polymers 2020, 12, 1820. https://doi.org/10.3390/polym12081820
Jouyandeh M, Ganjali MR, Aghazadeh M, Habibzadeh S, Formela K, Saeb MR. Bulk-Surface Modification of Nanoparticles for Developing Highly-Crosslinked Polymer Nanocomposites. Polymers. 2020; 12(8):1820. https://doi.org/10.3390/polym12081820
Chicago/Turabian StyleJouyandeh, Maryam, Mohammad Reza Ganjali, Mustafa Aghazadeh, Sajjad Habibzadeh, Krzysztof Formela, and Mohammad Reza Saeb. 2020. "Bulk-Surface Modification of Nanoparticles for Developing Highly-Crosslinked Polymer Nanocomposites" Polymers 12, no. 8: 1820. https://doi.org/10.3390/polym12081820
APA StyleJouyandeh, M., Ganjali, M. R., Aghazadeh, M., Habibzadeh, S., Formela, K., & Saeb, M. R. (2020). Bulk-Surface Modification of Nanoparticles for Developing Highly-Crosslinked Polymer Nanocomposites. Polymers, 12(8), 1820. https://doi.org/10.3390/polym12081820