Improvement of PLLA Ductility by Blending with PVDF: Localization of Compatibilizers at Interface and Its Glycidyl Methacrylate Content Dependency
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Polymer Blends
2.3. Tensile Property Tests
2.4. Dynamic Mechanical Analysis (DMA)
2.5. Microstructure Characterization
2.6. Rheology Characterization
2.7. Particle Size of Statistics
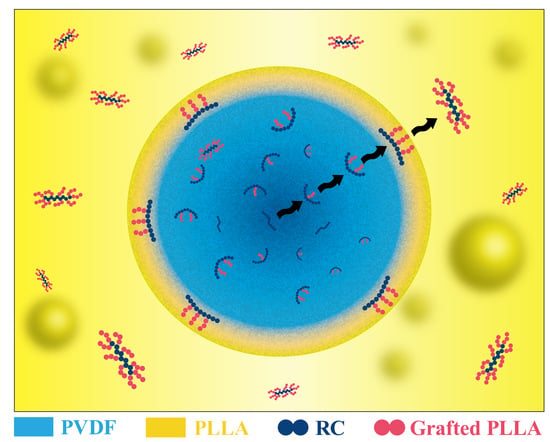
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Polylactic acid blends: The future of green, light and tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pan, M.W.; Li, Y.; Xu, J.Z.; Zhong, G.J.; Ji, X.; Yan, Z.; Li, Z.M. Core-shell nanoparticles toughened polylactide with excellent transparency and stiffness-toughness balance. Compos. Sci. Technol. 2018, 164, 168–177. [Google Scholar] [CrossRef]
- Deng, S.H.; Bai, H.W.; Liu, Z.W.; Zhang, Q.; Fu, Q. Toward supertough and heat-resistant stereocomplex-type polylactide/elastomer blends with impressive melt stability via in situ formation of graft copolymer during one-pot reactive melt blending. Macromolecules 2019, 52, 1718–1730. [Google Scholar] [CrossRef]
- Dong, W.Y.; He, M.F.; Wang, H.T.; Ren, F.L.; Zhang, J.Q.; Zhao, X.W.; Li, Y.J. PLLA/ABS blends compatibilized by reactive comb polymers: Double Tg depression and significantly improved toughness. ACS Sustain. Chem. Eng. 2015, 3, 2542–2550. [Google Scholar] [CrossRef]
- Dong, W.Y.; Jiang, F.H.; Zhao, L.P.; You, J.C.; Cao, X.J.; Li, Y.J. PLLA microalloys versus PLLA nanoalloys: Preparation, morphologies, and properties. ACS Appl. Mater. Inter. 2012, 4, 3667–3675. [Google Scholar] [CrossRef]
- Dong, W.Y.; Wang, H.T.; Ren, F.L.; Zhang, J.Q.; He, M.F.; Wu, T.; Li, Y.J. Dramatic improvement in toughness of PLLA/PVDF blends: The effect of compatibilizer architectures. ACS Sustain. Chem. Eng. 2016, 4, 4480–4489. [Google Scholar] [CrossRef]
- Oyama, H.T. Super-tough poly(lactic acid) materials: Reactive blending with ethylene copolymer. Polymer 2009, 50, 747–751. [Google Scholar] [CrossRef]
- Wang, Q.J.; Zhang, J.; Wang, X.H.; Wang, Z.G. Significant enhancement of notched Izod impact strength of PLA-based blends through encapsulating PA11 particles of low amounts by EGMA elastomer. Appl. Surf. Sci. 2020, 526, 146657. [Google Scholar] [CrossRef]
- Wu, B.G.; Zeng, Q.T.; Niu, D.Y.; Yang, W.J.; Dong, W.F.; Chen, M.Q.; Ma, P.M. Design of supertoughened and heat-resistant PLLA/elastomer blends by controlling the distribution of stereocomplex crystallites and the morphology. Macromolecules 2019, 52, 1092–1103. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.T.; Chen, J.L.; Fu, Z.A.; Zhao, X.W.; Li, Y.J. Copolymers containing two types of reactive groups: New compatibilizer for immiscible PLLA/PA11 polymer blends. Polymer 2019, 177, 139–148. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, F.; Yu, Q.L.; Ni, C.J.; Gu, X.Y.; Li, Y.J.; You, J.C. Fabrication of PLLA with high ductility and transparence by blending with tiny amount of PVDF and compatibilizers. Macromol. Mater. Eng. 2019, 304, 1900316. [Google Scholar] [CrossRef]
- Wang, H.T.; Fu, Z.A.; Dong, W.Y.; Li, Y.J.; Li, J.Y. Formation of interfacial janus nanomicelles by reactive blending and their compatibilization effects on immiscible polymer blends. J. Phys. Chem. B 2016, 120, 9240–9252. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, X.W.; Wang, H.T.; Chen, Q.; Wang, S.H.; Chen, Z.H.; Zhou, X.Y.; Fan, W.C.; Li, Y.J.; You, J.C. Sub-100 nm co-continuous structures fabricated in immiscible commodity polymer blend with extremely low volume/viscosity ratio. ACS Appl. Polym. Mater. 2019, 1, 124–129. [Google Scholar] [CrossRef]
- Jeon, H.K.; Zhang, J.; Macosko, C.W. Premade vs. reactively formed compatibilizers for PMMA/PS melt blends. Polymer 2005, 46, 12422–12429. [Google Scholar] [CrossRef]
- Koulic, C.; Yin, Z.; Pagnoulle, C.; Gilbert, B.; Jérôme, R. Premade versus in situ formed compatibilizer at the PS/PMMA interface: Contribution of the Raman confocal microscopy to the fracture analysis. Polymer 2001, 42, 2947–2957. [Google Scholar] [CrossRef] [Green Version]
- Macosko, C.; Guégan, P.; Khandpur, A. Compatibilizers for melt blending: Premade block copolymers. Macromolecules 1996, 29, 5590–5598. [Google Scholar] [CrossRef]
- Filippi, S.; Yordanov, H.; Minkova, L.; Polacco, G.; Talarico, M. Reactive compatibilizer precursors for LDPE/PA6 blends, 4: Maleic anhydride and glycidyl methacrylate grafted SEBS. Macromol. Mater. Eng. 2004, 289, 512–523. [Google Scholar] [CrossRef]
- Dong, W.Y.; Wang, H.T.; He, M.F.; Ren, F.L.; Wu, T.; Zheng, Q.R.; Li, Y.J. Synthesis of reactive comb polymers and their applications as a highly efficient compatibilizer in immiscible polymer blends. Ind. Eng. Chem. Res. 2015, 54, 2081–2089. [Google Scholar] [CrossRef]
- Fang, H.G.; Jiang, F.; Wu, Q.H.; Ding, Y.S.; Wang, Z.G. Supertough polylactide materials prepared through in situ reactive blending with PEG-based diacrylate monomer. ACS Appl. Mater. Inter. 2014, 6, 13552–13563. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.A.; Wang, H.T.; Zhao, X.W.; Horiuchi, S.; Li, Y.J. Immiscible polymer blends compatibilized with reactive hybrid nanoparticles: Morphologies and properties. Polymer 2017, 132, 353–361. [Google Scholar] [CrossRef]
- Kim, H.Y.; Ryu, D.Y.; Jeong, U.; Kho, D.H.; Kim, J.K. The effect of chain architecture of in situ formed copolymers on interfacial morphology of reactive polymer blends. Macromol. Rapid. Commun. 2005, 26, 1428–1433. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, H. The effect of PS—GMA as an in situ compatibilizer on the morphology and rheological properties of the immiscible PBT/PS blend. Polymer 1996, 37, 305–311. [Google Scholar] [CrossRef]
- Wei, B.; Lin, Q.Q.; Zheng, X.; Gu, X.Y.; Zhao, L.; Li, J.C.; Li, Y.J. Reactive splicing compatibilization of immiscible polymer blends: Compatibilizer synthesis in the melt state and compatibilizer architecture effects. Polymer 2019, 185, 121952. [Google Scholar] [CrossRef]
- Dong, W.Y.; Hakukawa, H.; Yamahira, N.; Li, Y.J.; Horiuchi, S. Mechanism of reactive compatibilization of PLLA/PVDF blends investigated by scanning transmission electron microscopy with energy-dispersive X-ray spectrometry and electron energy loss spectroscopy. ACS Appl. Polym. Mater. 2019, 1, 815–824. [Google Scholar] [CrossRef]
- Li, D.F.; Song, S.X.; Li, C.; Cao, C.L.; Sun, S.L.; Zhang, H.X. Compatibilization effect of MMA–co–GMA copolymers on the properties of polyamide 6/poly(vinylidene fluoride) blends. J. Polym. Res. 2015, 22, 102. [Google Scholar] [CrossRef]
- Mechbal, N.; Bousmina, M. Effect of copolymer addition on drop deformation during uniaxial elongation and during relaxation after cessation of flow. Macromolecules 2007, 40, 967–975. [Google Scholar] [CrossRef]
- Scott, C.E.; Macosko, C.W. Morphology development during reactive and non-reactive blending of an ethylene-propylene rubber with two thermoplastic matrices. Polymer 1994, 35, 5422–5433. [Google Scholar] [CrossRef]
- Sundararaj, U.; Macosko, C.; Rolando, R.; Chan, H. Morphology development in polymer blends. Polym. Eng. Sci. 1992, 32, 1814–1823. [Google Scholar] [CrossRef]
- Sundararaj, U.; Macosko, C.W. Drop breakup and coalescence in polymer blends: The effects of concentration and compatibilization. Macromolecules 1995, 28, 2647–2657. [Google Scholar] [CrossRef]
- Jermi, A.; He, Y.D.; Khan, Q.; Wahab, N. Barrier, mechanical, morphological and thermal properties of compatibilized high density polyethylene and polyamide 6 blends. Polym. Sci. Ser. B 2018, 60, 354–362. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Ma, Z.W.; Li, Y.Q.; Cui, J.; Jiang, W. Monte Carlo simulation of the compatibility of graft copolymer compatibilized two incompatible homopolymer blends: Effect of graft structure. J. Appl. Polym. Sci. 2007, 105, 1591–1596. [Google Scholar] [CrossRef]
- Scott, C.E.; Macosko, C.W. Model experiments concerning morphology development during the initial stages of polymer blending. Polym. Bull. 1991, 26, 341–348. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, J.X.; Jin, X.H.; Li, F.; Li, Y.J.; You, J.C. Direct evidence for the validity of assessing reaction extent by torque spectrum during reactive processing. Polymer 2020, 197, 122499. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Y.; Zhao, X.W.; Chen, Q.; Li, Y.J.; You, J.C. Graft ratio: Quantitative measurement and direct evidence for its blending sequence dependence during reactive compatibilization in PVDF/PLLA. Polymer 2019, 185, 121970. [Google Scholar] [CrossRef]
- Pan, P.J.; Shan, G.R.; Bao, Y.Z. Enhanced nucleation and crystallization of poly(l-lactic acid) by immiscible blending with poly(vinylidene fluoride). Ind. Eng. Chem. Res. 2014, 53, 3148–3156. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Kalló, A.; Kuleznev, V.N. Phase structure of impact-modified polypropylene blends. Polymer 1984, 25, 279–286. [Google Scholar] [CrossRef]
- Shi, T.F.; Ziegler, V.E.; Welge, I.C.; An, L.J.; Wolf, B.A. Evolution of the interfacial tension between polydisperse “immiscible” polymers in the absence and in the presence of a compatibilizer. Macromolecules 2004, 37, 1591–1599. [Google Scholar] [CrossRef]
- Walther, A.; Matussek, K.; Muller, A.H.E. Engineering nanostructured polymer blends with controlled nanoparticle location using Janus particles. ACS Nano 2008, 2, 1167–1178. [Google Scholar] [CrossRef]
- Martínez-Salazar, J.; Canalda, J.C.; Vallejo, B. Phase separation studies on poly(vinylidene fluoride) and poly(methyl methacrylate) quenched blends. Macromol. Symp. 1994, 78, 95–104. [Google Scholar] [CrossRef]
- Canalda, J.C.; Hoffmann, T.; Martínez-Salazar, J. On the melting behaviour of polymer single crystals in a mixture with a compatible polymer: 1. Poly(vinylidene fluoride)/poly(methyl methacrylate) blends. Polymer 1995, 36, 981–985. [Google Scholar] [CrossRef]
- Mijovic, J.; Sy, J.W.; Kwei, T.K. Reorientational dynamics of dipoles in poly(vinylidene fluoride)/poly(methyl methacrylate) (PVDF/PMMA) blends by dielectric spectroscopy. Macromolecules 1997, 30, 3042–3050. [Google Scholar] [CrossRef]
- Chen, D.P.; Wang, H.T.; Li, Y.J. Reactive compatibilization: Formation of double-grafted copolymers by in situ binary grafting and their compatibilization effect. ACS Appl. Mater. Interfaces 2017, 9, 33091–33099. [Google Scholar] [CrossRef] [PubMed]
- Li, R.M.; Yu, W.; Zhou, C.X. Investigation of phase separation in a partially miscible polymer blend by rheology. J. Macromol. Sci. B 2007, 46, 1051–1062. [Google Scholar] [CrossRef]








| Sample | Mixing Time (min) | E (GPa) | σy (MPa) | σb (MPa) | ε (%) |
|---|---|---|---|---|---|
| PVDF\PLLA\MG01 (5\95\3) | 10 min | 1.0 ± 0.1 | 51.1 ± 2.6 | 46.7 ± 3.9 | 8 ± 0 |
| 20 min | 1.2 ± 0.1 | 60.2 ± 0.1 | 45.4 ± 12.9 | 13 ± 3 | |
| 30 min | 1.1 ± 0.3 | 62.3 ± 0.8 | 42.1 ± 11.3 | 19 ± 6 | |
| 40 min | 1.3 ± 0.1 | 61.5 ± 0.2 | 46.9 ± 13.2 | 14 ± 3 | |
| PVDF/PLLA/MG02 (5/95/3) | 10 min | 1.3 ± 0.0 | 58.6 ± 0.0 | 50.8 ± 9.2 | 8 ± 2 |
| 20 min | 1.1 ± 0.0 | 60.1 ± 3.2 | 32.9 ± 0.7 | 143 ± 57 | |
| 30 min | 1.2 ± 0.0 | 56.5 ± 0.6 | 39.1 ± 2.0 | 291 ± 29 | |
| 40 min | 1.3 ± 0.1 | 59.8 ± 0.0 | 52.5 ± 3.0 | 14 ± 10 | |
| PVDF/PLLA/MG03 (5/95/3) | 5 min | 1.1 ± 0.1 | / | 54.9 ± 1.6 | 10 ± 0 |
| 10 min | 1.0 ± 0.1 | 60.5 ± 1.4 | 42.8 ± 1.6 | 273 ± 25 | |
| 20 min | 1.2 ± 0.1 | 57.3 ± 1.2 | 31.6 ± 2.2 | 138 ± 44 | |
| 30 min | 1.2 ± 0.0 | / | 62.9 ± 0.8 | 7.7 ± 0 |
| Sample | Mixing Time (min) | ε (%) | Tg (°C) | Half Peak Width |
|---|---|---|---|---|
| PVDF/PLLA/MG02 (5/95/3) | 10 | 7.9 ± 1.5 | 69.7 | 11.3 |
| 20 | 142.7 ± 57.0 | 69.6 | 11.6 | |
| 30 | 290.7 ± 28.5 | 69.6 | 11.6 | |
| 40 | 14.1 ± 10.3 | 70.7 | 11.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Gu, X.; Ni, C.; Li, F.; Li, Y.; You, J. Improvement of PLLA Ductility by Blending with PVDF: Localization of Compatibilizers at Interface and Its Glycidyl Methacrylate Content Dependency. Polymers 2020, 12, 1846. https://doi.org/10.3390/polym12081846
Zhang Y, Gu X, Ni C, Li F, Li Y, You J. Improvement of PLLA Ductility by Blending with PVDF: Localization of Compatibilizers at Interface and Its Glycidyl Methacrylate Content Dependency. Polymers. 2020; 12(8):1846. https://doi.org/10.3390/polym12081846
Chicago/Turabian StyleZhang, Yan, Xiaoying Gu, Chunjun Ni, Fei Li, Yongjin Li, and Jichun You. 2020. "Improvement of PLLA Ductility by Blending with PVDF: Localization of Compatibilizers at Interface and Its Glycidyl Methacrylate Content Dependency" Polymers 12, no. 8: 1846. https://doi.org/10.3390/polym12081846
APA StyleZhang, Y., Gu, X., Ni, C., Li, F., Li, Y., & You, J. (2020). Improvement of PLLA Ductility by Blending with PVDF: Localization of Compatibilizers at Interface and Its Glycidyl Methacrylate Content Dependency. Polymers, 12(8), 1846. https://doi.org/10.3390/polym12081846