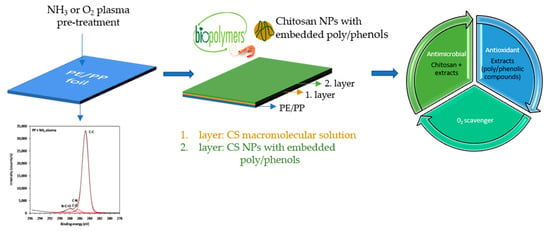
Two-Layer Functional Coatings of Chitosan Particles with Embedded Catechin and Pomegranate Extracts for Potential Active Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Solutions
2.3. Preparation of Chitosan Nanoparticles (CSNPs) with Embedded Extract (Catechin/Pomegranate)
2.4. Functionalization of PE and PP Surface with Macromolecular Chitosan Solution and CSNPs-Extracts’ Dispersion
2.4.1. Plasma Pretreatment
2.4.2. Deposition of Macromolecular Chitosan Solution and CSNPs-Extracts’ Dispersion
2.5. Dispersions’ Characterization
2.5.1. Particle Size and Electrokinetic Properties
2.5.2. Determination of Minimal Inhibitory Concentration (MIC)
2.6. Surface Elemental Composition of Functionalized Foils: ATR-FTIR Spectroscopy
2.7. Surface Elemental Composition of Functionalized Foils: XPS Analysis
2.8. Oxygen Permeability
2.9. Goniometry
2.10. Bioactivity
2.10.1. Antimicrobial Activity
2.10.2. Antioxidative Activity (ABTS Assay)
2.11. Polyelectrolyte Titration
3. Results and Discussion
3.1. Dispersions’ Characterization
3.1.1. Particle Size, PDI, and Zeta Potential Determination
3.1.2. Minimal Inhibitory Concentration (MIC)
3.2. Functional Foils
3.2.1. ATR-FTIR Spectroscopy
3.2.2. XPS Analysis
3.2.3. Oxygen Permeability
3.2.4. Goniometry
3.3. Bioactivity
3.3.1. Antimicrobial Activity
3.3.2. Anti-Oxidative Activity—ABTS Assay
3.4. Comparison of all Established Systems and the Most Promising Concept
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active packaging applications for food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [Green Version]
- Licciardello, F. Packaging, blessing in disguise. Review on its diverse contribution to food sustainability. Trends Food Sci. Technol. 2017, 65, 32–39. [Google Scholar] [CrossRef]
- Liljestrand, K. Logistics solutions for reducing food waste. Int. J. Phys. Distrib. Logist. Manag. 2017, 47, 318–339. [Google Scholar] [CrossRef]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Kovačević, D.B.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active packaging films with natural antioxidants to be used in meat industry: A review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef]
- Umaraw, P.; Munekata, P.E.; Verma, A.K.; Barba, F.J.; Singh, V.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M.C. State of the art of antimicrobial edible coatings for food packaging applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Lagarón, J.-M. Multifunctional and nanoreinforced polymers for food packaging. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–28. [Google Scholar]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable polymers and green-based antimicrobial packaging materials: A mini-review. Adv. Ind. Eng. Polym. Res. 2020, 3, 27–35. [Google Scholar] [CrossRef]
- Sofi, S.; Singh, J.; Rafiq, S.; Ashraf, U.; Dar, B.; Nayik, G.A. A comprehensive review on antimicrobial packaging and its use in food packaging. Curr. Nutr. Food Sci. 2018, 14, 305–312. [Google Scholar] [CrossRef]
- Cadar, C. Nanomaterials used in antimicrobial, active and intelligent food packaging. Carpathian J. Food Sci. Technol. 2014, 6, 47–57. [Google Scholar]
- Contini, C.; Katsikogianni, M.G.; O’Neill, F.T.; O’Sullivan, M.; Dowling, D.P.; Monahan, F.J. Development of active packaging containing natural antioxidants. Procedia Food Sci. 2011, 1, 224–228. [Google Scholar] [CrossRef] [Green Version]
- Katalinic, V.; Milos, M.; Kulisic, T.; Jukic, M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006, 94, 550–557. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Gao, H.-X.; He, Z.; Sun, Q.; He, Q.; Zeng, W.-C. A functional polysaccharide film forming by pectin, chitosan, and tea polyphenols. Carbohydr. Polym. 2019, 215, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango leaf extract incorporated chitosan antioxidant film for active food packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar]
- Salarbashi, D.; Tajik, S.; Ghasemlou, M.; Shojaee-Aliabadi, S.; Noghabi, M.S.; Khaksar, R. Characterization of soluble soybean polysaccharide film incorporated essential oil intended for food packaging. Carbohydr. Polym. 2013, 98, 1127–1136. [Google Scholar] [CrossRef]
- Ahvenainen, R. Novel Food Packaging Techniques; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Carrizo, D.; Gullo, G.; Bosetti, O.; Nerín, C. Development of an active food packaging system with antioxidant properties based on green tea extract. Food Addit. Contam. Part A 2014, 31, 364–373. [Google Scholar] [CrossRef]
- Duran, M.; Aday, M.S.; Zorba, N.N.D.; Temizkan, R.; Büyükcan, M.B.; Caner, C. Potential of antimicrobial active packaging ‘containing natamycin, nisin, pomegranate and grape seed extract in chitosan coating’to extend shelf life of fresh strawberry. Food Bioprod. Process. 2016, 98, 354–363. [Google Scholar] [CrossRef]
- Jonaidi Jafari, N.; Kargozari, M.; Ranjbar, R.; Rostami, H.; Hamedi, H. The effect of chitosan coating incorporated with ethanolic extract of propolis on the quality of refrigerated chicken fillet. J. Food Process. Preserv. 2018, 42, e13336. [Google Scholar] [CrossRef]
- Petrou, S.; Tsiraki, M.; Giatrakou, V.; Savvaidis, I. Chitosan dipping or oregano oil treatments, singly or combined on modified atmosphere packaged chicken breast meat. Int. J. Food Microbiol. 2012, 156, 264–271. [Google Scholar] [CrossRef]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Food 4 Future. Available online: http://www.food4future.net/en/ (accessed on 15 June 2020).
- Glaser, T.K.; Plohl, O.; Vesel, A.; Ajdnik, U.; Ulrih, N.P.; Hrnčič, M.K.; Bren, U.; Fras Zemljič, L. Functionalization of polyethylene (PE) and polypropylene (PP) material using chitosan nanoparticles with incorporated resveratrol as potential active packaging. Materials 2019, 12, 2118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potrč, S.; Fras Zemljič, L.; Sterniša, M.; Smole Možina, S.; Plohl, O. Development of Biodegradable Whey-Based Laminate Functionalised by Chitosan—Natural Extract Formulations. Int. J. Mol. Sci. 2020, 21, 3668. [Google Scholar] [CrossRef] [PubMed]
- Potrč, S.; Sterniša, M.; Smole Možina, S.; Knez Hrnčič, M.; Fras Zemljič, L. Bioactive Characterization of Packaging Foils Coated by Chitosan and Polyphenol Colloidal Formulations. Int. J. Mol. Sci. 2020, 21, 2610. [Google Scholar] [CrossRef] [Green Version]
- Zemljič, L.F.; Plohl, O.; Vesel, A.; Luxbacher, T.; Potrč, S. Physicochemical Characterization of Packaging Foils Coated by Chitosan and Polyphenols Colloidal Formulations. Int. J. Mol. Sci. 2020, 21, 495. [Google Scholar] [CrossRef] [Green Version]
- Hegemann, D.; Brunner, H.; Oehr, C. Plasma treatment of polymers for surface and adhesion improvement. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2003, 208, 281–286. [Google Scholar] [CrossRef]
- Klančnik, A.; Piskernik, S.; Jeršek, B.; Možina, S.S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods 2010, 81, 121–126. [Google Scholar] [CrossRef]
- Dickson, D.; Liu, G.; Li, C.; Tachiev, G.; Cai, Y. Dispersion and stability of bare hematite nanoparticles: Effect of dispersion tools, nanoparticle concentration, humic acid and ionic strength. Sci. Total Environ. 2012, 419, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Alexandre, E.M.; Silva, S.; Santos, S.A.; Silvestre, A.J.; Duarte, M.F.; Saraiva, J.A.; Pintado, M. Antimicrobial activity of pomegranate peel extracts performed by high pressure and enzymatic assisted extraction. Food Res. Int. 2019, 115, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Pagliarulo, C.; De Vito, V.; Picariello, G.; Colicchio, R.; Pastore, G.; Salvatore, P.; Volpe, M.G. Inhibitory effect of pomegranate (Punica granatum L.) polyphenol extracts on the bacterial growth and survival of clinical isolates of pathogenic Staphylococcus aureus and Escherichia coli. Food Chem. 2016, 190, 824–831. [Google Scholar] [CrossRef]
- Surendhiran, D.; Li, C.; Cui, H.; Lin, L. Fabrication of high stability active nanofibers encapsulated with pomegranate peel extract using chitosan/PEO for meat preservation. Food Packag. Shelf Life 2020, 23, 100439. [Google Scholar] [CrossRef]
- Taylor, P.W.; Hamilton-Miller, J.M.; Stapleton, P.D. Antimicrobial properties of green tea catechins. Food Sci. Technol. Bull. 2005, 2, 71. [Google Scholar] [CrossRef] [Green Version]
- Nuamsetti, T.; Dechayuenyong, P.; Tantipaibulvut, S. Antibacterial activity of pomegranate fruit peels and arils. Sci. Asia 2012, 38, 319–322. [Google Scholar] [CrossRef] [Green Version]
- Fernandes Queiroz, M.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef]
- Li, P.; Dai, Y.-N.; Zhang, J.-P.; Wang, A.-Q.; Wei, Q. Chitosan-alginate nanoparticles as a novel drug delivery system for nifedipine. Int. J. Biomed. Sci. 2008, 4, 221. [Google Scholar]
- Oliveira, R.N.; Mancini, M.C.; Oliveira, F.C.S.d.; Passos, T.M.; Quilty, B.; Thiré, R.M.d.S.M.; McGuinness, G.B. FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Matéria (Rio de Jan.) 2016, 21, 767–779. [Google Scholar] [CrossRef] [Green Version]
- Hoseyni, S.Z.; Jafari, S.M.; Tabarestani, H.S.; Ghorbani, M.; Assadpour, E.; Sabaghi, M. Production and characterization of catechin-loaded electrospun nanofibers from Azivash gum-polyvinyl alcohol. Carbohydr. Polym. 2020, 235, 115979. [Google Scholar] [CrossRef]
- Tkavc, T.; Petrinič, I.; Luxbacher, T.; Vesel, A.; Ristić, T.; Zemljič, L.F. Influence of O2 and CO2 plasma treatment on the deposition of chitosan onto polyethylene terephthalate (PET) surfaces. Int. J. Adhes. Adhes. 2014, 48, 168–176. [Google Scholar] [CrossRef]
- Vesel, A.; Kolar, M.; Doliska, A.; Stana-Kleinschek, K.; Mozetic, M. Etching of polyethylene terephthalate thin films by neutral oxygen atoms in the late flowing afterglow of oxygen plasma. Surf. Interface Anal. 2012, 44, 1565–1571. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetic, M. New developments in surface functionalization of polymers using controlled plasma treatments. J. Phys. D Appl. Phys. 2017, 50, 293001. [Google Scholar] [CrossRef]
- Vukušić, T.; Vesel, A.; Holc, M.; Ščetar, M.; Jambrak, A.R.; Mozetič, M. Modification of physico-chemical properties of acryl-coated polypropylene foils for food packaging by reactive particles from oxygen plasma. Materials 2018, 11, 372. [Google Scholar] [CrossRef] [Green Version]
- Miklavčič Višnjevec, A.; Ota, A.; Skrt, M.; Butinar, B.; Smole Možina, S.; Gunde Cimerman, N.; Nečemer, M.; Baruca Arbeiter, A.; Hladnik, M.; Krapac, M. Genetic, biochemical, nutritional and antimicrobial characteristics of pomegranate (Punica granatum L.) grown in Istria. Food Technol. Biotechnol. 2017, 55, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.; Schoenweitz, C.; Langowski, H.C. Thin laminate films for barrier packaging application–influence of down gauging and substrate surface properties on the permeation properties. Packag. Technol. Sci. 2012, 25, 137–148. [Google Scholar] [CrossRef]
- Struller, C.; Kelly, P.; Copeland, N. Aluminum oxide barrier coatings on polymer films for food packaging applications. Surf. Coat. Technol. 2014, 241, 130–137. [Google Scholar] [CrossRef]
- Introzzi, L.; Fuentes-Alventosa, J.M.; Cozzolino, C.A.; Trabattoni, S.; Tavazzi, S.; Bianchi, C.L.; Schiraldi, A.; Piergiovanni, L.; Farris, S. “Wetting enhancer” pullulan coating for antifog packaging applications. ACS Appl. Mater. Interfaces 2012, 4, 3692–3700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ristić, T.; Lasič, S.; Kosalec, I.; Bračič, M.; Fras-Zemljič, L. The effect of chitosan nanoparticles onto Lactobacillus cells. React. Funct. Polym. 2015, 97, 56–62. [Google Scholar] [CrossRef]
- Fathima, A.; Rao, J.R. Selective toxicity of Catechin—A natural flavonoid towards bacteria. Appl. Microbiol. Biotechnol. 2016, 100, 6395–6402. [Google Scholar] [CrossRef]
- Fan, F.-Y.; Sang, L.-X.; Jiang, M. Catechins and their therapeutic benefits to inflammatory bowel disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Elfalleh, W.; Hannachi, H.; Tlili, N.; Yahia, Y.; Nasri, N.; Ferchichi, A. Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J. Med. Plants Res. 2012, 6, 4724–4730. [Google Scholar] [CrossRef]
- European Commission. Union Guidelines on Regulation (EU) No 10/2011 on Plastic Materials and Articles Intended to Come into Contact with Food; European Commission: Brussels, Belgium, 2013.






| Sample Notation | Description of the Samples |
|---|---|
| PE | polyethylene |
| PP | polypropylene |
| PE-NH3 | PE treated with NH3 plasma |
| PP-NH3 | PP treated with NH3 plasma |
| PE-O2 | PE treated with O2 plasma |
| PP-O2 | PP treated with O2 plasma |
| CSNPs | chitosan nanoparticles |
| CSNPs POM | chitosan nanoparticles with embedded pomegranate extract |
| CSNPs CAT | chitosan nanoparticles with embedded catechin |
| PE (2%CS, CSNPs POM) | untreated PE, coated with 2% CS-1.layer and CSNPs POM-2.layer |
| PP (2%CS, CSNPs POM) | untreated PP, coated with 2% CS-1.layer and CSNPs POM-2.layer |
| PE-NH3 (2%CS, CSNPs POM) | PE treated with NH3 plasma, coated with 2% CS-1.layer and CSNPs POM-2.layer |
| PP-NH3 (2%CS, CSNPs POM) | PP treated with NH3 plasma, coated with 2% CS-1.layer and CSNPs POM-2.layer |
| PE-O2 (2%CS, CSNPs POM) | PE treated with O2 plasma, coated with 2% CS-1.layer and CSNPs POM-2.layer |
| PP-O2 (2%CS, CSNPs POM) | PP treated with O2 plasma, coated with 2% CS-1.layer and CSNPs POM-2.layer |
| PE (2%CS, CSNPs CAT) | untreated PE, coated with 2% CS-1.layer and CSNPs CAT-2.layer |
| PP (2%CS, CSNPs CAT) | untreated PP, coated with 2% CS-1.layer and CSNPs CAT-2.layer |
| PE-NH3 (2%CS, CSNPs CAT) | PE treated with NH3 plasma, coated with 2% CS-1.layer and CSNPs CAT-2.layer |
| PP-NH3 (2%CS, CSNPs CAT) | PP treated with NH3 plasma, coated with 2% CS-1.layer and CSNPs CAT-2.layer |
| PE-O2 (2%CS, CSNPs CAT) | PE treated with O2 plasma, coated with 2% CS-1.layer and CSNPs CAT-2.layer |
| PP-O2 (2%CS, CSNPs CAT) | PP treated with O2 plasma, coated with 2% CS-1.layer and CSNPs CAT-2.layer |
| Sample | Z-Average (nm) by Intensity | PDI | ZP (mV) | pH |
|---|---|---|---|---|
| CSNPs | 358 ± 40 | 0.831 | 36 ± 5 | 4 |
| CSNPs POM | 893 ± 227 | 0.836 | 16 ± 7 | 4 |
| CSNPs CAT | 833 ± 138 | 1.000 | 20 ± 4 | 4 |
| Bacteria | MIC (mg·mL−1) of POM | MIC (mg·mL−1) of CAT |
|---|---|---|
| Escherichia coli | 1.56 | 10.00 |
| Staphylococcus aureus | 0.20 | 2.50 |
| Composition/(at. %) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Untreated Foils | NH3 Plasma Pretreatment | O2 Plasma Pretreatment | |||||||
| Sample | C | N | O | C | N | O | C | N | O |
| PE | 98.9 | / | 1.1 | 90.3 | 4.5 | 5.5 | 86.3 | / | 13.7 |
| PP | 98.7 | / | 1.3 | 94.0 | 3.6 | 4.3 | 85.3 | / | 14.7 |
| PE (2% CS, CSNPs POM) | 80.1 | 3.4 | 16.4 | 72.0 | 4.1 | 22.8 | 60.5 | 5.8 | 33.8 |
| PP (2% CS, CSNPs POM) | 89.7 | 2.0 | 8.3 | 88.1 | 2.2 | 9.1 | 61.9 | 5.4 | 32.7 |
| Differences between functionalized | −18.8 | 3.4 | 15.4 | −18.3 | −0.4 | 17.3 | −25.8 | 5.8 | 20.1 |
| and reference foils (%) | −9.0 | 2.0 | 7.0 | −5.9 | −1.4 | 4.8 | −23.4 | 5.4 | 18.0 |
| PE (2% CS, CSNPs CAT) | 74.9 | 3.2 | 21.0 | 85.3 | 2.8 | 11.9 | 68.4 | 3.7 | 26.2 |
| PP (2% CS, CSNPs CAT) | 91.8 | 1.3 | 7.0 | 72.6 | 3.1 | 23.0 | 69.1 | 3.9 | 25.7 |
| Differences between functionalized | −24.0 | 3.2 | 9.9 | −5.0 | −1.7 | 6.4 | −17.9 | 3.7 | 12.5 |
| and reference foils (%) | −6.9 | 1.3 | 5.7 | −21.4 | −0.5 | 18.7 | −16.2 | 3.9 | 11.0 |
| Sample | OTR (cm3/m2·24h) | OTR (%) | Permeability Reduced (%) |
|---|---|---|---|
| PE | 3226 ± 62 | 100 ± 2 | / |
| PP | 1078 ± 36 | 100 ± 3 | / |
| PE (2% CS, CSNP POM) | 757 ± 64 | 23 ± 2 | 77 ± 2 |
| PP (2% CS, CSNP POM) | 666 ± 19 | 62 ± 4 | 38 ± 4 |
| PE-NH3 (2% CS, CSNP POM) | 458 ± 28 | 14 ± 2 | 86 ± 2 |
| PP-NH3 (2% CS, CSNP POM) | 373 ± 17 | 35 ± 3 | 65 ± 3 |
| PE-O2 (2% CS, CSNP POM) | 36 ± 6 | 1 ± 1 | 99 ± 1 |
| PP-O2 (2% CS, CSNP POM) | 19 ± 1 | 2 ± 1 | 98 ± 1 |
| PE (2% CS, CSNP CAT) | 2459 ± 81 | 76 ± 4 | 24 ± 4 |
| PP (2% CS, CSNP CAT) | 975 ± 41 | 90 ± 6 | 10 ± 6 |
| PE-NH3 (2% CS, CSNP CAT) | 646 ± 28 | 20 ± 2 | 80 ± 2 |
| PP-NH3 (2% CS, CSNP CAT) | 645 ± 21 | 60 ± 4 | 40 ± 4 |
| PE-O2 (2% CS, CSNP CAT) | 19 ± 2 | 1 ± 1 | 99 ± 1 |
| PP-O2 (2% CS, CSNP CAT) | 23 ± 1 | 2 ± 1 | 98 ± 1 |
| Sample | Average Angle (α/°) | Difference (%) |
|---|---|---|
| PE | 108.2 ± 3.1 | / |
| PP | 109.3 ± 2.9 | / |
| PE-NH3 | 101.5 ± 2.3 | 6.2 ± 2.3 |
| PP-NH3 | 103.3 ± 1.3 | 5.5 ± 1.3 |
| PE-O2 | 29.7 ± 3.2 | 72.6 ± 3.2 |
| PP-O2 | 37.0 ± 1.8 | 66.1 ± 1.8 |
| PE (2% CS, CSNPs POM) | 87.5 ± 2.5 | 19.1 ± 2.5 |
| PP (2% CS, CSNPs POM) | 85.9 ± 1.2 | 21.4 ± 1.2 |
| PE-NH3 (2% CS, CSNPs POM) | 85.6 ± 1.8 | 20.9 ± 1.8 |
| PP-NH3 (2% CS, CSNPs POM) | 75.3 ± 2.1 | 31.1 ± 2.1 |
| PE-O2 (2% CS, CSNPs POM) | 47.0 ± 2.5 | 56.6 ± 2.5 |
| PP-O2 (2% CS, CSNPs POM) | 55.7 ± 3.2 | 49.0 ± 3.2 |
| PE (2% CS, CSNPs CAT) | 85.7 ± 1.7 | 20.8 ± 1.7 |
| PP (2% CS, CSNPs CAT) | 87.9 ± 2.2 | 19.6 ± 2.2 |
| PE-NH3 (2% CS, CSNPs CAT) | 75.2 ± 1.3 | 30.5 ± 1.3 |
| PP-NH3 (2% CS, CSNPs CAT) | 80.8 ± 2.2 | 26.1 ± 2.2 |
| PE-O2 (2% CS, CSNPs CAT) | 37.1 ± 1.7 | 65.7 ± 1.7 |
| PP-O2 (2% CS, CSNPs CAT) | 41.6 ± 2.8 | 61.9 ± 2.8 |
| Bacteria | Staphylococcus aureus | Escherichia coli | ||
|---|---|---|---|---|
| Sample | Number of Cells | Antimicrobial | Number of Cells | Antimicrobial |
| (log cfu/cm2) | Efficacy (%) | (log cfu/cm2) | Efficacy (%) | |
| PE | 4.58 ± 0.06 | 4.58 ± 0.06 | ||
| PP | 5.10 ± 0.05 | 5.10 ± 0.05 | ||
| PE (2% CS, CSNP POM) | 3.00 ± 0.43 | 34.47 ± 9.38 | 3.99 ± 0.51 | 23.44 ± 9.73 |
| PP (2% CS, CSNP POM) | 1.01 ± 0.39 | 78.63 ± 9.69 | 3.40 ± 0.23 | 34.49 ± 4.22 |
| PE-NH3 (2% CS, CSNP POM) | <1 | >90 | 2.27 ± 0.11 | 55.96 ± 2.16 |
| PP-NH3 (2% CS, CSNP POM) | 1.81 ± 0.24 | 67.49 ± 4.27 | 3.14 ± 0.38 | 39.10 ± 7.33 |
| PE-O2 (2% CS, CSNP POM) | <1 | >90 | 1.37 ± 0.03 | 75.82 ± 2.15 |
| PP-O2 (2% CS, CSNP POM) | <1 | >90 | 1.30 ± 0.16 | 75.03 ± 3.04 |
| PE (2% CS, CSNP CAT) | <1 | >90 | 2.92 ± 0.07 | 29.54 ± 2.90 |
| PP (2% CS, CSNP CAT) | <1 | >90 | 2.03 ± 0.20 | 51.44 ± 4.89 |
| PE-NH3 (2% CS, CSNP CAT) | <1 | >90 | 2.01 ± 0.05 | 57.22 ± 1.16 |
| PP-NH3 (2% CS, CSNP CAT) | <1 | >90 | 1.39 ± 0.14 | 70.71 ± 3.06 |
| PE-O2 (2% CS, CSNP CAT) | <1 | >90 | 3.28 ± 0.08 | 30.28 ± 1.65 |
| PP-O2 (2% CS, CSNP CAT) | 0.83 ± 0.67 | 83.78 ± 13.05 | 2.15 ± 0.28 | 54.30 ± 5.93 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potrč, S.; Kraševac Glaser, T.; Vesel, A.; Poklar Ulrih, N.; Fras Zemljič, L. Two-Layer Functional Coatings of Chitosan Particles with Embedded Catechin and Pomegranate Extracts for Potential Active Packaging. Polymers 2020, 12, 1855. https://doi.org/10.3390/polym12091855
Potrč S, Kraševac Glaser T, Vesel A, Poklar Ulrih N, Fras Zemljič L. Two-Layer Functional Coatings of Chitosan Particles with Embedded Catechin and Pomegranate Extracts for Potential Active Packaging. Polymers. 2020; 12(9):1855. https://doi.org/10.3390/polym12091855
Chicago/Turabian StylePotrč, Sanja, Tjaša Kraševac Glaser, Alenka Vesel, Nataša Poklar Ulrih, and Lidija Fras Zemljič. 2020. "Two-Layer Functional Coatings of Chitosan Particles with Embedded Catechin and Pomegranate Extracts for Potential Active Packaging" Polymers 12, no. 9: 1855. https://doi.org/10.3390/polym12091855
APA StylePotrč, S., Kraševac Glaser, T., Vesel, A., Poklar Ulrih, N., & Fras Zemljič, L. (2020). Two-Layer Functional Coatings of Chitosan Particles with Embedded Catechin and Pomegranate Extracts for Potential Active Packaging. Polymers, 12(9), 1855. https://doi.org/10.3390/polym12091855