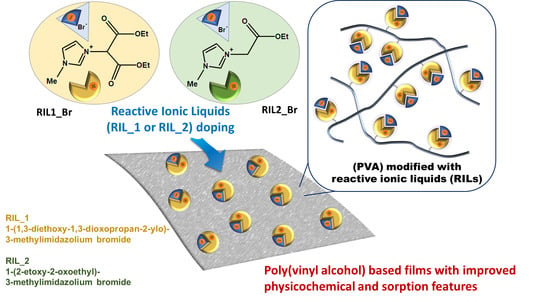
The Impact of Reactive Ionic Liquids Addition on the Physicochemical and Sorption Properties of Poly(Vinyl Alcohol)-Based Films
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Reactive Ionic Liquid (RIL) Synthesis
2.3. Elaboration of PVA-RIL Films
2.4. Membrane Characterization
2.5. The Measurements of Sorption Processes in Water (H2O), Ethanol (EtOH), and Propan-2-ol (IPA)
3. Results and Discussion
3.1. Physicochemical Properties of PVA-RIL Films
3.1.1. RILs and PVA Characterization
3.1.2. Thermal Properties
3.1.3. Morphology and Surface Characterization
3.1.4. Mechanical Performance
3.2. Membrane Swelling Behavior in Water (H2O), Ethanol (EtOH), and Propan-2-ol (IPA)
3.2.1. Degree of Swelling in Water (H2O), Ethanol (EtOH), and Propan-2-ol (IPA)
3.2.2. Kinetics Analysis of Water Sorption Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xi, T.; Lu, Y.; Ai, X.; Tang, L.; Yao, L.; Hao, W.; Cui, P. Ionic liquid copolymerized polyurethane membranes for pervaporation separation of benzene/cyclohexane mixtures. Polymer 2019, 185, 121948. [Google Scholar] [CrossRef]
- Sajjad, Z.; Gilani, M.A.; Nizami, A.-S.; Bilad, M.R.; Khan, A.L. Development of novel hydrophilic ionic liquid membranes for the recovery of biobutanol through pervaporation. J. Environ. Manag. 2019, 251, 109618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.; Liu, Q.; Li, W.; Xing, W. Fabrication of ionic liquids-functionalized PVA catalytic composite membranes to enhance esterification by pervaporation. J. Membr. Sci. 2019, 584, 268–281. [Google Scholar] [CrossRef]
- Dmitrenko, M.E.; Penkova, A.V.; Kuzminova, A.I.; Morshed, M.; Larionov, M.I.; Alem, H.; Zolotarev, A.A.; Ermakov, S.S.; Roizard, D. Investigation of new modification strategies for PVA membranes to improve their dehydration properties by pervaporation. Appl. Surf. Sci. 2018, 450, 527–537. [Google Scholar] [CrossRef]
- Dai, Z.; Deng, J.; Ansaloni, L.; Janakiram, S.; Deng, L. Thin-film-composite hollow fiber membranes containing amino acid salts as mobile carriers for CO2 separation. J. Membr. Sci. 2019, 578, 61–68. [Google Scholar] [CrossRef]
- Jahan, Z.; Niazi, M.B.K.; Hägg, M.-B.; Gregersen, Ø.W. Decoupling the effect of membrane thickness and CNC concentration in PVA based nanocomposite membranes for CO2/CH4 separation. Sep. Purif. Technol. 2018, 578, 220–225. [Google Scholar] [CrossRef]
- Guerrero, G.; Venturi, D.; Peters, T.; Rival, N.; Denonville, C.; Simon, C.; Henriksen, P.P.; Hägg, M.-B. Influence of Functionalized Nanoparticles on the CO2/N2 Separation Properties of PVA-based Gas Separation Membranes. Energy Procedia 2017, 114, 627–635. [Google Scholar] [CrossRef]
- Torstensen, J.Ø.; Helberg, R.M.L.; Deng, L.; Gregersen, Ø.W.; Syverud, K. PVA/nanocellulose nanocomposite membranes for CO2 separation from flue gas. Int. J. Greenh. Gas Con. 2019, 81, 93–102. [Google Scholar] [CrossRef]
- Klepić, M.; Setničková, K.; Lanč, M.; Žák, M.; Izák, P.; Dendisová, M.; Fuoco, A.; Jansen, J.C.; Friess, K. Permeation and sorption properties of CO2-selective blend membranes based on polyvinyl alcohol (PVA) and 1-ethyl-3-methylimidazolium dicyanamide ([EMIM][DCA]) ionic liquid for effective CO2/H2 separation. J. Membr. Sci. 2019, 597, 117623. [Google Scholar]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Barooah, M.; Mandal, B. Synthesis, characterization and CO2 separation performance of novel PVA/PG/ZIF-8 mixed matrix membrane. J. Membr. Sci. 2019, 572, 198–209. [Google Scholar] [CrossRef]
- Dilshad, M.R.; Islam, A.; Hamidullah, U.; Jamshaid, F.; Ahmad, A.; Butt, M.T.Z.; Ijaz, A. Effect of alumina on the performance and characterization of cross-linked PVA/PEG 600 blended membranes for CO2/N2 separation. Sep. Purif. Technol. 2019, 210, 627–635. [Google Scholar] [CrossRef]
- Zhang, S.; Zou, Y.; Wei, T.; Mu, C.; Liu, X.; Tong, Z. Pervaporation dehydration of binary and ternary mixtures of n-butyl acetate, n-butanol and water using PVA-CS blended membranes. Sep. Purif. Technol. 2017, 173, 314–322. [Google Scholar] [CrossRef]
- Penkova, A.V.; Acquah, S.F.A.; Dmitrenko, M.E.; Sokolova, M.P.; Mikhailova, M.E.; Polyakov, E.S.; Ermakov, S.S.; Markelov, D.A.; Roizard, D. Improvement of pervaporation PVA membranes by the controlled incorporation of fullerenol nanoparticles. Mater. Des. 2016, 96, 416–423. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Wang, Q.; Ma, J.; Cao, J.; Hu, W.; Wu, Z. Relationship between polymers compatibility and casting solution stability in fabricating PVDF/PVA membranes. J. Membr. Sci. 2017, 537, 263–271. [Google Scholar] [CrossRef]
- Helberg, R.M.L.; Dai, Z.; Ansaloni, L.; Deng, L. PVA/PVP blend polymer matrix for hosting carriers in facilitated transport membranes: Synergistic enhancement of CO2 separation performance. Green Energy Environ. 2020, 5, 59–68. [Google Scholar] [CrossRef]
- Dilshad, M.R.; Islam, A.; Sabir, A.; Shafiq, M.; Butt, M.T.Z.; Ijaz, A.; Jamil, T. Fabrication and performance characterization of novel zinc oxide filled cross-linked PVA/PEG 600 blended membranes for CO2/N2 separation. J. Ind. Eng. Chem. 2017, 55, 65–73. [Google Scholar] [CrossRef]
- Chaudhari, S.; Kwon, Y.; Shon, M.; Nam, S.; Park, Y. Surface-modified polyvinyl alcohol (PVA) membranes for pervaporation dehydration of epichlorohydrin (ECH), isopropanol (IPA), and water ternary feed mixtures. J. Ind. Eng. Chem. 2020, 81, 185–195. [Google Scholar] [CrossRef]
- Bojnourd, F.M.; Pakizeh, M. Preparation and characterization of a nanoclay/PVA/PSf nanocomposite membrane for removal of pharmaceuticals from water. Appl. Clay Sci. 2018, 162, 326–338. [Google Scholar] [CrossRef]
- Selim, A.; Toth, A.J.; Haaz, E.; Fozer, D.; Szanyi, A.; Hegyesi, N.; Mizsey, P. Preparation and characterization of PVA/GA/Laponite membranes to enhance pervaporation desalination performance. Sep. Purif. Technol. 2019, 221, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Liang, Y.; Miao, J.; Wu, B.; Hossain, M.M.; Cao, M.; Ge, Q.; Su, L.; Zheng, Z.; Yang, B.; et al. Preparation and properties of polyvinyl alcohol (PVA)/mesoporous silica supported phosphotungstic acid (MS-HPW) hybrid membranes for alkali recovery. J. Membr. Sci. 2019, 592, 117388. [Google Scholar] [CrossRef]
- Aparicio, G.M.; Vargas, R.A.; Bueno, P.R. Protonic conductivity and thermal properties of cross-linked PVA/TiO2 nanocomposite polymer membranes. J. Non-Cryst. Solids 2019, 522, 119520. [Google Scholar] [CrossRef]
- Jarosik, A.; Krajewski, S.R.; Lewandowski, A.; Radzimski, P. Conductivity of ionic liquids in mixtures. J. Mol. Liq. 2006, 123, 43–50. [Google Scholar] [CrossRef]
- Mannan, H.A.; Mukhtar, H.; Shahrun, M.S.; Bustam, M.A.; Man, Z.; Bakar, M.Z.A. Effect of [EMIM][Tf2N] Ionic Liquid on Ionic Liquid-polymeric Membrane (ILPM) for CO2/CH4 Separation. Procedia Eng. 2016, 148, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.K.; Kim, N.H.; Lee, J.H. Effects of ionic liquid-functionalized mesoporous silica on the proton conductivity of acid-doped poly(2,5-benzimidazole) composite membranes for high-temperature fuel cells. J. Membr. Sci. 2014, 449, 136–145. [Google Scholar] [CrossRef]
- Ilyas, A.; Muhammad, N.; Gilani, M.A.; Ayub, K.; Vankelecom, I.F.J.; Khan, A.L. Supported protic ionic liquid membrane based on 3-(trimethoxysilyl)propan-1-aminium acetate for the highly selective separation of CO2. J. Membr. Sci. 2017, 543, 301–309. [Google Scholar] [CrossRef]
- Vatani, M.; Raisi, A.; Pazuki, G. Three-component mixed matrix membrane containing [Hmim][PF6] ionic liquid and ZSM-5 nanoparticles based on poly (ether-block-amide) for the pervaporation process. J. Mol. Liq. 2019, 277, 471–480. [Google Scholar] [CrossRef]
- Lam, B.; Wei, M.; Zhu, L.; Luo, S.; Guo, R.; Morisato, A.; Alexandridis, P.; Lin, H. Cellulose triacetate doped with ionic liquids for membrane gas separation. Polymer 2016, 89, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mohshim, D.F.; Mukhtar, H.; Man, Z. A study on carbon dioxide removal by blending the ionic liquid in membrane synthesis. Sep. Purif. Technol. 2018, 196, 20–26. [Google Scholar] [CrossRef]
- Rynkowska, E.; Fatyeyeva, K.; Kujawa, J.; Dzieszkowski, K.; Wolan, A.; Kujawski, W. The effect of reactive ionic liquid or plasticizer incorporation on the physicochemical and transport properties of cellulose acetate propionate-based membranes. Polymers 2018, 10, 86. [Google Scholar] [CrossRef] [Green Version]
- Zia-ul-Mustafa, M.; Mukhtar, H.; Nordin, N.; Mannan, H.A. Effect of imidazolium based ionic liquids on PES membrane for CO2/CH4 separation. Mater. Today Proc. 2019, 16, 1976–1982. [Google Scholar] [CrossRef]
- Saroj, A.L.; Singh, R.K. Thermal, dielectric and conductivity studies on PVA/Ionic liquid [EMIM][EtSO4] based polymer electrolytes. J. Phys. Chem. Solids 2012, 73, 162–168. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Wang, J.; Wang, L. Synthesized Geminal-imidazolium-type ionic liquids applying for PVA-FP/[DimL][OH] anion exchange membranes for fuel cells. Polymer 2019, 170, 31–42. [Google Scholar] [CrossRef]
- Saroj, A.L.; Krishnamoorthi, S.; Singh, R.K. Structural, thermal and electrical transport behaviour of polymer electrolytes based on PVA and imidazolium based ionic liquid. J. Non-Cryst. Solids 2017, 473, 87–95. [Google Scholar] [CrossRef]
- Hussan, K.P.S.; Thayyil, M.S.; Jinitha, T.V.; Kolte, J. Development of an ionogel membrane PVA/[EMIM] [SCN] with enhanced thermal stability and ionic conductivity for electrochemical application. J. Mol. Liq. 2019, 274, 402–413. [Google Scholar] [CrossRef]
- Kujawa, J.; Rynkowska, E.; Fatyeyeva, K.; Knozowska, K.; Wolan, A.; Dzieszkowski, K.; Li, G.; Kujawski, W. Preparation and Characterization of Cellulose Acetate Propionate Films Functionalized with Reactive Ionic Liquids. Polymers 2019, 11, 1217. [Google Scholar] [CrossRef] [Green Version]
- Rynkowska, E.; Fatyeyeva, K.; Marais, S.; Kujawa, J.; Kujawski, W. Chemically and Thermally Crosslinked PVA-Based Membranes: Effect on Swelling and Transport Behavior. Polymers 2019, 11, 1799. [Google Scholar] [CrossRef] [Green Version]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- Gindl, M.; Sinn, G.; Gindl, W.; Reiterer, A.; Tschegg, S. A comparison of different methods to calculate the surface free energy of wood using contact angle measurements. Colloids Surf. A 2001, 181, 279–287. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Kujawa, J.; Rozicka, A.; Cerneaux, S.; Kujawski, W. The influence of surface modification on the physicochemical properties of ceramic membranes. Colloids Surf. A 2014, 443, 567–575. [Google Scholar] [CrossRef]
- Chan, L.W.; Hao, J.S.; Heng, P.W.S. Evaluation of permeability and mechanical properties of composite polyvinyl alcohol films. Chem. Pharm. Bull. 1999, 47, 1412–1416. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Felipe, A.; Moliner-Estopiñán, C.; Imrie, C.T.; Ribes-Greus, A. Characterization of crosslinked poly(vinyl alcohol)-based membranes with different hydrolysis degrees for their use as electrolytes in direct methanol fuel cells. J. Appl. Polym. Sci. 2012, 124, 1000–1011. [Google Scholar]
- Gui, H.; Li, Y.; Chen, S.; Xu, P.; Zheng, B.; Ding, Y. Effects of biodegradable imidazolium-based ionic liquid with ester group on the structure and properties of PLLA. Macromol. Res. 2014, 22, 583–591. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, H.J.; Stafford, C.M. Thermoplastic elastomers based on ionic liquid and poly(vinyl alcohol). Macromolecules 2011, 44, 2170–2178. [Google Scholar] [CrossRef]
- Dong, K.; Liu, X.; Dong, H.; Zhang, X.; Zhang, S. Multiscale studies on ionic liquids. Chem. Rev. 2017, 117, 6636–6695. [Google Scholar] [CrossRef] [PubMed]
- Donato, K.; Matějka, L.; Mauler, R.; Donato, R. Recent applications of ionic liquids in the sol-gel process for polymer–silica nanocomposites with ionic interfaces. Colloids Surf. 2018, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- Rozik, N.N.; Ward, A.A. A novel approach on poly(ionic liquid)-based poly(vinyl alcohol) as a hydrophilic/hydrophobic conductive polymer electrolytes. Polym. Bull. 2018, 75, 267–287. [Google Scholar] [CrossRef]
- Patachia, S.; Friedrich, C.; Florea, C.; Croitoru, C. Study of the PVA hydrogel behaviour in 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid. Express Polym. Lett. 2011, 5, 197–207. [Google Scholar] [CrossRef]
- Weber, R.L.; Ye, Y.; Banik, S.M.; Elabd, Y.A.; Hickner, M.A.; Mahanthappa, M.K. Thermal and ion transport properties of hydrophilic and hydrophobic polymerized styrenic imidazolium ionic liquids. J. Polym. Sci. Part B 2011, 49, 1287–1296. [Google Scholar] [CrossRef]
- Ohtani, H.; Ishimura, S.; Kumai, M. Thermal decomposition behaviors of imidazolium-type ionic liquids studied by pyrolysis-gas chromatography. Anal. Sci. 2008, 24, 1335–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krapcho, A.P.; Ciganek, E. The Krapcho Dealkoxycarbonylation Reaction of Esters with α-Electron-Withdrawing Substituents. In Organic Reactions; Denmark, S.E., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 1–536. [Google Scholar]
- Garcia, M.T.; Ribosa, I.; Perez, L.; Manresa, A.; Comelles, F. Aggregation behavior and antimicrobial activity of ester-functionalized imidazolium- and pyridinium-based ionic liquids in aqueous solution. Langmuir 2013, 29, 2536–2545. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Bai, L.; Zeng, S.; Zhang, X.; Nie, Y.; Deng, L.; Zhang, S. Ether-functionalized ionic liquid based composite membranes for carbon dioxide separation. RSC Adv. 2016, 6, 45184–45192. [Google Scholar] [CrossRef]
- Hooshyari, K.; Javanbakht, M.; Adibi, M. Novel composite membranes based on dicationic ionic liquid and polybenzimidazole mixtures as strategy for enhancing thermal and electrochemical properties of proton exchange membrane fuel cells applications at high temperature. Int. J. Hydrogen Energy 2016, 41, 10870–10883. [Google Scholar] [CrossRef]
- Gimenes, M.L.; Liu, L.; Feng, X. Sericin/poly(vinyl alcohol) blend membranes for pervaporation separation of ethanol/water mixtures. J. Membr. Sci. 2007, 295, 71–79. [Google Scholar] [CrossRef]
- Sajjan, A.M.; Kariduraganavar, M.Y. Development of novel membranes for PV separation of water–isopropanol mixtures using poly(vinyl alcohol) and gelatin. J. Membr. Sci. 2013, 438, 8–17. [Google Scholar] [CrossRef]
- Rynkowska, E.; Dzieszkowski, K.; Lancien, A.; Fatyeyeva, K.; Szymczyk, A.; Kujawa, J.; Koter, S.; Marais, S.; Wolan, A.; Kujawski, W. Physicochemical properties and pervaporation performance of dense membranes based on cellulose acetate propionate (CAP) and containing polymerizable ionic liquid (PIL). J. Membr. Sci. 2017, 544, 243–251. [Google Scholar] [CrossRef]
- Kujawski, J.K.; Kujawski, W.M.; Sondej, H.; Jarzynka, K.; Kujawska, A.; Bryjak, M.; Rynkowska, E.; Knozowska, K.; Kujawa, J. Dewatering of 2,2,3,3-tetrafluoropropan-1-ol by hydrophilic pervaporation with poly(vinyl alcohol) based Pervap™ membranes. Sep. Purif. Technol. 2017, 174, 520–528. [Google Scholar] [CrossRef]
- Chaudhari, S.; Kwon, Y.; Moon, M.; Shon, M.; Park, Y.; Nam, S. Melamine-modified silicotungstic acid incorporated into the polyvinyl alcohol/polyvinyl amine blend membrane for pervaporation dehydration of water/isopropanol mixtures. Vacuum 2018, 147, 115–125. [Google Scholar] [CrossRef]
- Kujawski, W.; Poźniak, G. Swelling properties of ion-exchange membranes in contact with water–alcohol mixtures. Sep. Sci. Technol. 2005, 39, 2137–2154. [Google Scholar] [CrossRef]
- Kujawski, W.; Poźniak, G.; Nguyen, Q.T.; Néel, J. Properties of interpolymer PESS ion-exchange membranes in contact with solvents of different polarities. Sep. Sci. Technol. 1997, 32, 1657–1667. [Google Scholar] [CrossRef]
- Rynkowska, E.; Kujawa, J.; Chappey, C.; Fatyeyeva, K.; Karpenko-Jereb, L.; Kelterer, A.-M.; Marais, S.; Kujawski, W. Effect of the polar–nonpolar liquid mixtures on pervaporative behavior of perfluorinated sulfonic membranes in lithium form. J. Membr. Sci. 2016, 518, 313–327. [Google Scholar] [CrossRef]
- Robati, D. Pseudo-second-order kinetic equations for modeling adsorption systems for removal of lead ions using multi-walled carbon nanotube. J. Nanostruct. Chem. 2013, 3, 55. [Google Scholar] [CrossRef] [Green Version]
- Sabarish, R.; Unnikrishnan, G. PVA/PDADMAC/ZSM-5 zeolite hybrid matrix membranes for dye adsorption: Fabrication, characterization, adsorption, kinetics and antimicrobial properties. J. Environ. Chem. Eng. 2018, 6, 3860–3873. [Google Scholar] [CrossRef]













| Absorption Bands | Wavenumber [cm−1] | |
|---|---|---|
| RIL1_Br [30] | RIL2_Br | |
| Water presence in a sample | - | 3425 |
| C–H stretching (imidazolium ring) | 3070 | 3082 |
| C–H stretching (alkyl) | 2993 | 2982 |
| C=O stretching (–COO– ester group) | 1759 | 1745 |
| Imidazolium ring stretching | 1556 | 1572 |
| C–O stretching (–COO– ester group) | 1221, 1263 | 1225 |
| C–H scissoring deformation (CH2 in methylene group) | 1471 | 1470 |
| C–H asymmetric deformation (CH3 group) | 1444 | 1439 |
| H–C–C and H–C–N bending (imidazolium ring) | 1182 | 1174 |
| C–H in-plane deformation (imidazolium ring) | 839 | 876 |
| C–H out-of-plane deformation (imidazolium ring) | 750 | 766 |
| Membranes | Degradation Temperature [°C] | |
|---|---|---|
| at 5% Weight Loss | at 10% Weight Loss | |
| PVA | 163 ± 2 | 243 ± 2 |
| RIL1_Br | 97 ± 2 | 118 ± 2 |
| PVA-9-RIL1_Br | 183 ± 2 | 251 ± 2 |
| PVA-16.7-RIL1_Br | 126 ± 2 | 234 ± 2 |
| PVA-23-RIL1_Br | 120 ± 2 | 235 ± 2 |
| RIL2_Br | 90 ± 2 | 161 ± 2 |
| PVA-9-RIL2_Br | 148 ± 2 | 250 ± 2 |
| PVA-16.7-RIL2_Br | 122 ± 2 | 242 ± 2 |
| PVA-23-RIL2_Br | 126 ± 2 | 228 ± 2 |
| Parameters | Rq [nm] | Ra [nm] |
|---|---|---|
| Scanning Area | 5 μm × 5 μm | |
| PVA | 3.2 ± 0.8 | 2.3 ± 0.5 |
| PVA-9-RIL1_Br | 14.0 ± 1.3 | 10.2 ± 1.1 |
| PVA-16.7-RIL1_Br | 7.5 ± 2.7 | 5.6 ± 2.6 |
| PVA-23-RIL1_Br | 4.4 ± 2.5 | 2.7 ± 1.7 |
| PVA-9-RIL2_Br | 7.9 ± 3.4 | 6.5 ± 2.8 |
| PVA-16.7-RIL2_Br | 6.8 ± 3.2 | 5.1 ± 2.4 |
| PVA-23-RIL2_Br | 6.3 ± 1.1 | 5.1 ± 0.8 |
| Membranes | DSe (%) | ||
|---|---|---|---|
| Water | Ethanol | Propan-2-ol | |
| Pristine PVA | 98.6 | 3.2 | 4.7 |
| PVA-9-RIL1_Br | 137.7 | 0.8 | 2.0 |
| PVA-16.7-RIL1_Br | 141.0 | 2.9 | 1.9 |
| PVA-23-RIL1_Br | 216.4 | 2.8 | 2.2 |
| PVA-33-RIL1_Br | 348.9 | 2.9 | 2.6 |
| PVA-9-RIL2_Br | 95.7 | 1.2 | 0.6 |
| PVA-16.7-RIL2_Br | 108.5 | 0.8 | 1.2 |
| PVA-23-RIL2_Br | 512.3 | 0.7 | 2.7 |
| Kinetics Models | Non-Linear Equation | Linear Equation | ||
|---|---|---|---|---|
| PFO | ) | (3) | (4) | |
| PSO | (5) | (6) | ||
| PVA-RIL1_Br | Parameters | RIL1_Br (wt %) | RIL2_Br (wt %) | |||||
|---|---|---|---|---|---|---|---|---|
| 9 | 16.7 | 23 | 33 | 9 | 16.7 | 23 | ||
| Experiment | DSe (%) | 138 | 141 | 216 | 349 | 96 | 109 | 512 |
| FO (non-linear mode) | DSe (%) | 135 | 136 | 210 | 350 | 94 | 103 | 515 |
| k1 (s−1) | 4.22 × 10−3 | 6.21 × 10−3 | 1.26 × 10−3 | 8.27 × 10−4 | 1.58 × 10−3 | 2.34 × 10−3 | 9.07 × 10−4 | |
| R2 | 0.979 | 0.987 | 0.992 | 0.991 | 0.994 | 0.988 | 0.993 | |
| PFO (linear mode) | DSe (%) | 58 | 46 | 184 | 378 | 94 | 94 | 524 |
| k1 (s−1) | 6.91 × 10−4 | 9.21 × 10−4 | 9.21 × 10−4 | 9.21 × 10−4 | 1.44 × 10−3 | 1.36 × 10−3 | 1.10 × 10−3 | |
| R2 | 0.716 | 0.546 | 0986 | 0.982 | 0.994 | 0.983 | 0.994 | |
| PSO (non-linear mode) | DSe (%) | 146 | 145 | 245 | 423 | 109 | 117 | 652 |
| k2 (s−1) | 4.00 × 10−5 | 5.95 × 10−5 | 5.97 × 10−6 | 2.09 × 10−6 | 1.71 × 10−5 | 2.47 × 10−5 | 1.32 × 10−6 | |
| R2 | 0.980 | 0.993 | 0.995 | 0.994 | 0.994 | 0.995 | 0.983 | |
| PSO (linear mode) | DSe (%) | 142 | 145 | 238 | 416 | 128 | 125 | 714 |
| k2 (s−1) | 4.37 × 10−5 | 5.15 × 10−5 | 6.96 × 10−6 | 2.51 × 10−6 | 8.10 × 10−6 | 1.63 × 10−6 | 8.11 × 10−7 | |
| R2 | 0.999 | 0.999 | 0.997 | 0.987 | 0.879 | 0.972 | 0.944 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Rynkowska, E.; Fatyeyeva, K.; Kujawa, J.; Dzieszkowski, K.; Wolan, A.; Marais, S.; Chappey, C.; Rafiński, Z.; Kujawski, W. The Impact of Reactive Ionic Liquids Addition on the Physicochemical and Sorption Properties of Poly(Vinyl Alcohol)-Based Films. Polymers 2020, 12, 1958. https://doi.org/10.3390/polym12091958
Li G, Rynkowska E, Fatyeyeva K, Kujawa J, Dzieszkowski K, Wolan A, Marais S, Chappey C, Rafiński Z, Kujawski W. The Impact of Reactive Ionic Liquids Addition on the Physicochemical and Sorption Properties of Poly(Vinyl Alcohol)-Based Films. Polymers. 2020; 12(9):1958. https://doi.org/10.3390/polym12091958
Chicago/Turabian StyleLi, Guoqiang, Edyta Rynkowska, Kateryna Fatyeyeva, Joanna Kujawa, Krzysztof Dzieszkowski, Andrzej Wolan, Stephane Marais, Corinne Chappey, Zbigniew Rafiński, and Wojciech Kujawski. 2020. "The Impact of Reactive Ionic Liquids Addition on the Physicochemical and Sorption Properties of Poly(Vinyl Alcohol)-Based Films" Polymers 12, no. 9: 1958. https://doi.org/10.3390/polym12091958
APA StyleLi, G., Rynkowska, E., Fatyeyeva, K., Kujawa, J., Dzieszkowski, K., Wolan, A., Marais, S., Chappey, C., Rafiński, Z., & Kujawski, W. (2020). The Impact of Reactive Ionic Liquids Addition on the Physicochemical and Sorption Properties of Poly(Vinyl Alcohol)-Based Films. Polymers, 12(9), 1958. https://doi.org/10.3390/polym12091958