Enhanced Thermal Conductivity of Silicone Composites Filled with Few-Layered Hexagonal Boron Nitride
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of FL-hBN Powder
2.2. Preparation of h-BN/silicone and FL-hBN/silicone Composites
3. Characterizations
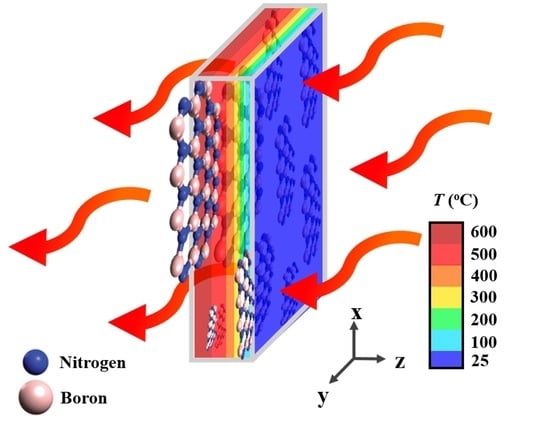
4. Results and Discussion
- AR = Aspect ratio,
- D = The diameter of the platelets (average particle size),
- t = The thickness of the platelets,
- S = The specific surface area of the particles,
- ρ = The density of the platelets.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sim, L.C.; Ramanan, S.R.; Ismail, H.; Seetharamu, K.N.; Goh, T.J. Thermal characterization of Al2O3 and ZnO reinforced silicone rubber as thermal pads for heat dissipation purposes. Thermochim. Acta 2005, 43, 155–165. [Google Scholar] [CrossRef]
- Gao, N.; Liu, W.; Yan, Z.; Wang, Z. Synthesis and properties of transparent cycloaliphatic epoxy–silicone resins for opto-electronic devices packaging. Opt. Mater. 2013, 35, 567–575. [Google Scholar] [CrossRef]
- Chen, C.; Tang, Y.; Ye, Y.S.; Xue, Z.; Xue, Y.; Xie, X.; Mai, Y.-W. High-performance epoxy/silica coated silver nanowire composites as underfill material for electronic packaging. Compos. Sci. Technol. 2014, 105, 80–85. [Google Scholar] [CrossRef]
- Fang, L.; Wu, W.; Huang, X.; He, J.; Jiang, P. Hydrangea-like zinc oxide superstructures for ferroelectric polymer composites with high thermal conductivity and high dielectric constant. Compos. Sci. Technol. 2015, 107, 67–74. [Google Scholar] [CrossRef]
- Song, H.; Park, K.H.; Kim, B.H.; Choi, Y.W.; Jun, G.H.; Lee, D.J.; Kong, B.S.; Paik, K.W.; Jeon, S. Enhanced thermal conductivity of epoxy–graphene composites by using non-oxidized graphene flakes with non-covalent functionalization. Adv. Mater. 2012, 25, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.-C.; Ma, C.-C.M.; Lu, C.-H.; Yang, S.-Y.; Lee, S.-H.; Hsiao, M.-C.; Yen, M.-Y.; Chiou, K.-C.; Lee, T.-M. Thermal conductivity and structure of non-covalent functionalized graphene/epoxy composites. Carbon 2011, 49, 5107–5116. [Google Scholar] [CrossRef]
- Bjorneklett, A.; Halbo, L.; Kristiansen, H. Thermal conductivity of epoxy adhesives filled with silver particles. Int. J. Adhes. Adhes. 1992, 12, 99–104. [Google Scholar] [CrossRef]
- Papanicolaou, G.C.; Theocaris, P.S. Thermal properties and volume fraction of the boundary interphase in metal-filled epoxies. Colloid Polym. Sci. 1979, 257, 239–246. [Google Scholar] [CrossRef]
- Huang, X.; Iizuka, T.; Jiang, P.; Ohki, Y.; Tanaka, T. Role of interface on the thermal conductivity of highly filled dielectric epoxy/AlN composites. J. Phys. Chem. C 2012, 116, 13629–13639. [Google Scholar] [CrossRef]
- Yu, H.; Li, L.; Kido, T.; Xi, G.; Xu, G.; Guo, F. Thermal and insulating properties of epoxy/aluminum nitride composites used for thermal interface material. J. Appl. Polym. Sci. 2012, 124, 669–677. [Google Scholar] [CrossRef]
- Im, H.; Kim, J. The effect of Al2O3 doped multi-walled carbon nanotubes on the thermal conductivity of Al2O3/epoxy terminated poly (dimethylsiloxane) composites. Carbon 2011, 49, 3503–3511. [Google Scholar] [CrossRef]
- Gao, Z.; Zhao, L. Effect of nano-fillers on the thermal conductivity of epoxy composites with micro-Al2O3 particles. Mater. Des. 2015, 66, 176–182. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, X.; Liu, X.; Xiong, D. Improved thermal conductivity of epoxy composites using a hybrid multi-walled carbon nanotube/micro-SiC filler. Carbon 2010, 48, 1171–1176. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, X.; Gu, M.; Xiong, D. Study on mechanical; thermal and electrical characterizations of nano-SiC/epoxy composites. Polym. J. 2009, 41, 51. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, D.; Zou, X.; Mao, L.; Shi, L. The exfoliation and functionalization of boron nitride nanosheets and their utilization in silicone composites with improved thermal conductivity. J. Mater. Sci. Mater. Electron. 2017, 28, 12984–12994. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.H.; Lindsay, L.; Cherns, D.; Pomeroy, J.W.; Liu, S.; Edgar, J.H.; Kuball, M. Modulating the thermal conductivity in hexagonal boron nitride via controlled boron isotope concentration. Commun. Phys. 2019, 2, 1–8. [Google Scholar] [CrossRef]
- Hou, J.; Li, G.; Yang, N.; Qin, L.; Grami, M.E.; Zhang, Q.; Wang, N.; Qu, X. Preparation and characterization of surface modified boron nitride epoxy composites with enhanced thermal conductivity. RSC Adv. 2014, 4, 44282–44290. [Google Scholar] [CrossRef]
- Muratov, D.S.; Kuznetsov, D.V.; Il’Inykh, I.A.; Burmistrov, I.N.; Mazov, I.N. Thermal conductivity of polypropylene composites filled with silane-modified hexagonal BN. Compos. Sci. Technol. 2015, 111, 40–43. [Google Scholar] [CrossRef]
- Lee, G.-W.; Park, M.; Kim, J.; Lee, J.I.; Yoon, H.G. Enhanced thermal conductivity of polymer composites filled with hybrid filler. Compos. Part A Appl. Sci. Manuf. 2006, 37, 727–734. [Google Scholar] [CrossRef]
- Liu, M.-S.; Lin, M.C.-C.; Huang, I.-T.; Wang, C.-C. Enhancement of thermal conductivity with carbon nanotube for nanofluids. Int. Commun. Heat Mass Transf. 2005, 32, 1202–1210. [Google Scholar] [CrossRef]
- Gantenbein, D.; Schoelkopf, J.; Matthews, G.P.; Gane, P.A. Determining the size distribution-defined aspect ratio of platy particles. Appl. Clay Sci. 2011, 53, 544–552. [Google Scholar] [CrossRef]
- Pacile, D.; Meyer, J.C.; Girit, Ç.Ö.; Zettl, A. The two-dimensional phase of boron nitride: Few-atomic-layer sheets and suspended membranes. Appl. Phys. Lett. 2008, 92, 133107. [Google Scholar] [CrossRef]
- Li, L.H.; Glushenkov, A.M.; Hait, S.K.; Hodgson, P.; Chen, Y. High-efficient production of boron nitride nanosheets via an optimized ball milling process for lubrication in oil. Sci. Rep. 2014, 4, 7288. [Google Scholar]
- Zhu, K.; Wang, W.; Meng, A.; Zhao, M.; Wang, J.; Zhao, M.; Zhang, D.; Jia, Y.; Xu, C.; Li, Z. Mechanically exfoliated g-C3N4 thin nanosheets by ball milling as high performance photocatalysts. RSC Adv. 2015, 5, 56239–56243. [Google Scholar] [CrossRef]
- An, S.-J.; Kim, Y.H.; Lee, C.; Park, D.Y.; Jeong, M.S. Exfoliation of Transition Metal Dichalcogenides by a High-Power Femtosecond Laser. Sci. Rep. 2018, 8, 12957. [Google Scholar] [CrossRef] [PubMed]
- Rafie-Sarmazdeh, Z.; Jafari, S.H.; Ahmadi, S.J.; Zahedi-Dizaji, S.M. Large-scale exfoliation of hexagonal boron nitride with combined fast quenching and liquid exfoliation strategies. J. Mater. Sci. 2016, 51, 3162–3169. [Google Scholar] [CrossRef]
- Güler, Ö.; Güler, S.H. Production of graphene–boron nitride hybrid nanosheets by liquid-phase exfoliation. Optik 2016, 127, 4630–4634. [Google Scholar] [CrossRef]
- Gerchman, D.; Alves, A.K. Solution-processable exfoliation and suspension of atomically thin WSe2. J. Colloid Interface Sci. 2016, 468, 247–252. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Ambrosi, A.; Eng, A.Y.S.; Sofer, Z.; Pumera, M. Transition metal dichalcogenides (MoS2, MoSe2, WS2 and WSe2) exfoliation technique has strong influence upon their capacitance. Electrochem. Commun. 2015, 56, 24–28. [Google Scholar] [CrossRef]
- Jeon, J.; Jang, S.K.; Jeon, S.M.; Yoo, G.; Jang, Y.H.; Park, J.-H.; Lee, S. Layer-controlled CVD growth of large-area two-dimensional MoS2 films. Nanoscale 2015, 7, 1688–1695. [Google Scholar] [CrossRef]
- Perea-López, N.; Lin, Z.; Pradhan, N.R.; Iñiguez-Rábago, A.; Elías, A.L.; McCreary, A.; Lou, J.; Ajayan, P.M.; Terrones, H.; Balicas, L. CVD-grown monolayered MoS2 as an effective photosensor operating at low-voltage. 2D Mater. 2014, 1, 011004. [Google Scholar] [CrossRef]
- Fan, D.; Feng, J.; Liu, J.; Gao, T.; Ye, Z.; Chen, M.; Lv, X. Hexagonal boron nitride nanosheets exfoliated by sodium hypochlorite ball mill and their potential application in catalysis. Ceram. Int. 2016, 42, 7155–7163. [Google Scholar] [CrossRef]
- Wu, X.F.; Zhao, Z.-h.; Sun, Y.; Li, H.; Zhang, C.-X.; Wang, Y.-J.; Zheng, S.-S.; Zhang, H. Few-layer boron nitride nanosheets: Preparation; characterization and application in epoxy resin. Ceram. Int. 2017, 43, 2274–2278. [Google Scholar] [CrossRef]
- Nacken, T.; Damm, C.; Walter, J.; Rüger, A.; Peukert, W. Delamination of graphite in a high pressure homogenizer. RSC Adv. 2015, 5, 57328–57338. [Google Scholar] [CrossRef] [Green Version]
- Skaltsas, T.; Ke, X.; Bittencourt, C.; Tagmatarchis, N. Ultrasonication induces oxygenated species and defects onto exfoliated graphene. J. Phys. Chem. C 2013, 117, 23272–23278. [Google Scholar] [CrossRef]
- Liang, S.; Shen, Z.; Yi, M.; Liu, L.; Zhang, X.; Cai, C.; Ma, S. Effects of Processing Parameters on Massive Production of Graphene by Jet Cavitation. J. Nanosci. Nanotechnol. 2015, 15, 2686–2694. [Google Scholar] [CrossRef]
- Lin, P.-C.; Wu, J.-Y.; Liu, W.-R. Green and facile synthesis of few-layer graphene via liquid exfoliation process for Lithium-ion batteries. Sci. Rep. 2018, 8, 9766. [Google Scholar] [CrossRef]
- Yeh, Y.-Y.; Chiang, W.-H.; Liu, W.-R. Synthesis of few-layer WS2 by jet cavitation as anode material for lithium ion batteries. J. Alloy. Compd. 2019, 775, 1251–1258. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Liu, W.-R. Few-layered MoSe2 ultrathin nanosheets as anode materials for lithium ion batteries. J. Alloys Compd. 2020, 831, 152074. [Google Scholar] [CrossRef]
- Cai, W.; Hong, N.; Feng, X.; Zeng, W.; Shi, Y.; Zhang, Y.; Wang, B.; Hu, Y. A facile strategy to simultaneously exfoliate and functionalize boron nitride nanosheets via Lewis acid-base interaction. Chem. Eng. J. 2017, 330, 309–321. [Google Scholar] [CrossRef]
- Lei, W.; Mochalin, V.N.; Liu, D.; Qin, S.; Gogotsi, Y.; Chen, Y. Boron nitride colloidal solutions; ultralight aerogels and freestanding membranes through one-step exfoliation and functionalization. Nat. Commun. 2015, 6, 8849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, C.; Duan, B.; Li, L.; Xie, B.; Huang, M.; Luo, X.B. Thermal Conductivity of Polymer-Based Composites with Magnetic Aligned Hexagonal Boron Nitride Platelets. ACS Appl. Mater. Interfaces 2015, 7, 13000–13006. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.Y.; Liu, Y.; Raghavan, S.; Moon, K.-S.; Suresh, K.; Sitaraman, S.K.; Wong, C.-P. Magnetic Alignment of Hexagonal Boron Nitride Platelets in Polymer Matrix: Toward High Performance Anisotropic Polymer Composites for Electronic Encapsulation. ACS Appl. Mater. Interfaces 2013, 5, 7633–7640. [Google Scholar] [CrossRef] [PubMed]










| Fraction (wt.%) | Density (cm3 g−1) | Specific Heat Capacity (J g−1 K−1) | Thermal Diffusivity (mm2 s−1) | Thermal Conductivity (W m−1 K−1) | |
|---|---|---|---|---|---|
| Pure silicone | 0 | 1.030 | 1.993 | 0.119 | 0.244 |
| hBN/silicone | 10 | 1.089 | 1.928 | 0.164 | 0.344 |
| 20 | 1.141 | 1.386 | 0.226 | 0.357 | |
| 30 | 1.182 | 1.264 | 0.284 | 0.424 | |
| FL-hBN/silicone | 10 | 1.060 | 1.944 | 0.203 | 0.418 |
| 20 | 1.119 | 1.434 | 0.273 | 0.438 | |
| 30 | 1.170 | 1.381 | 0.319 | 0.515 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, W.-C.; Hsieh, Y.-T.; Liu, W.-R. Enhanced Thermal Conductivity of Silicone Composites Filled with Few-Layered Hexagonal Boron Nitride. Polymers 2020, 12, 2072. https://doi.org/10.3390/polym12092072
Cheng W-C, Hsieh Y-T, Liu W-R. Enhanced Thermal Conductivity of Silicone Composites Filled with Few-Layered Hexagonal Boron Nitride. Polymers. 2020; 12(9):2072. https://doi.org/10.3390/polym12092072
Chicago/Turabian StyleCheng, Wei-Cheng, Yi-Ting Hsieh, and Wei-Ren Liu. 2020. "Enhanced Thermal Conductivity of Silicone Composites Filled with Few-Layered Hexagonal Boron Nitride" Polymers 12, no. 9: 2072. https://doi.org/10.3390/polym12092072
APA StyleCheng, W. -C., Hsieh, Y. -T., & Liu, W. -R. (2020). Enhanced Thermal Conductivity of Silicone Composites Filled with Few-Layered Hexagonal Boron Nitride. Polymers, 12(9), 2072. https://doi.org/10.3390/polym12092072