Change in Convection Mixing Properties with Salinity and Temperature: CO2 Storage Application
Abstract
:1. Introduction
2. Materials and Methods
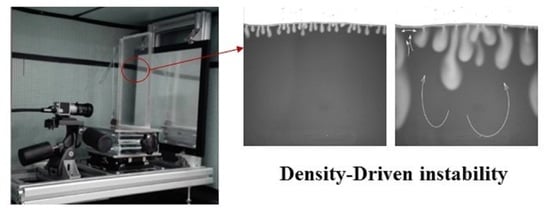
2.1. Experimental Apparatus and Materials
2.2. Experimental Procedures
2.3. Dimensionless Parameters
3. Results and Discussion
3.1. Convection Mixing Occurrence and Development
3.1.1. Diffusion-Dominated Stage and Convection Finger Formation
3.1.2. Convection Finger Development to the Shutdown
3.2. Effect of Brine Salinity on the Onset Time
3.3. Effect of Brine Salinity on Finger Development and Mixing
3.4. Effect of Temperature on the Onset Time and Convection Finger
3.5. Dissolved Mass CO2
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sarafraz, M.M.; Safaei, M.R. Diurnal thermal evaluation of an evacuated tube solar collector (ETSC) charged with graphene nanoplatelets-methanol nano-suspension. Renew. Energy 2019, 142, 364–372. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Goodarzi, M.; Tlili, I.; Alkanhal, T.A.; Arjomandi, M. Thermodynamic potential of a high-concentration hybrid photovoltaic/thermal plant for co-production of steam and electricity. J. Therm. Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- Goodarzi, H.; Akbari, O.A.; Sarafraz, M.M.; Karchegani, M.M.; Safaei, M.R.; Shabani, G.A.S. Numerical Simulation of Natural Convection Heat Transfer of Nanofluid With Cu, MWCNT, and Al2O3 Nanoparticles in a Cavity With Different Aspect Ratios. J. Therm. Sci. Eng. Appl. 2019, 11. [Google Scholar] [CrossRef]
- Anwar, M.N.; Fayyaz, A.; Sohail, N.F.; Khokhar, M.F.; Baqar, M.; Khan, W.D.; Rasool, K.; Rehan, M.; Nizami, A.S. CO(2) capture and storage: A way forward for sustainable environment. J. Environ. Manag. 2018, 226, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Song, Y.; Jiang, L.; Zhao, Y.; Ruan, X.; Zhang, Y.; Wang, S. Hydrate-based technology for CO2 capture from fossil fuel power plants. Appl. Energy 2014, 116, 26–40. [Google Scholar] [CrossRef]
- Viebahn, P.; Vallentin, D.; Hoeller, S. Prospects of carbon capture and storage (CCS) in India’s power sector—An integrated assessment. Appl. Energy 2014, 117, 62–75. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Xue, Z.; Park, H. Enhancement of CO2 dissolution and sweep efficiency in saline aquifer by micro bubble CO2 injection. Int. J. Heat Mass Transf. 2019, 138, 1211–1221. [Google Scholar] [CrossRef]
- Benson, S.M.; Orr, F.M., Jr. Carbon dioxide capture and storage. MRS Bull. 2008, 33, 303–305. [Google Scholar] [CrossRef] [Green Version]
- Bader, A.G.; Thibeau, S.; Vincke, O.; Jannaud, F.D.; Saysset, S.; Joffre, G.H.; Giger, F.M.; David, M.; Gimenez, M.; Dieulin, A.; et al. CO2 Storage Capacity Evaluation in Deep Saline Aquifers for an Industrial Pilot Selection. Methodology and Results of the France Nord Project. In Proceedings of the 12th International Conference on Greenhouse Gas Control Technologies, Ghgt-12, Austin, TX, USA, 5–9 October 2014; Dixon, T., Herzog, H., Twinning, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 63, pp. 2779–2788. [Google Scholar]
- Rathnaweera, T.D.; Ranjith, P.G.; Perera, M.S.A. Experimental investigation of geochemical and mineralogical effects of CO2 sequestration on flow characteristics of reservoir rock in deep saline aquifers. Sci. Rep. 2016, 6, 19362. [Google Scholar] [CrossRef] [Green Version]
- Soroush, M.; Wessel-Berg, D.; Torsaeter, O.; Kleppe, J. Investigating residual trapping in CO2 storage in saline aquifers—Application of a 2D glass model, and image analysis. Energy Sci. Eng. 2014, 2, 149–163. [Google Scholar] [CrossRef]
- Neufeld, J.A.; Hesse, M.A.; Riaz, A.; Hallworth, M.A.; Tchelepi, H.A.; Huppert, H.E. Convective dissolution of carbon dioxide in saline aquifers. Geophys. Res. Lett. 2010, 37. [Google Scholar] [CrossRef]
- Sainz-Garcia, A.; Abarca, E.; Nardi, A.; Grandia, F.; Oelkers, E.H. Convective mixing fingers and chemistry interaction in carbon storage. Int. J. Greenh. Gas Control 2017, 58, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.; Zhou, Q.; Birkholzer, J.T. Early detection of brine and CO2 leakage through abandoned wells using pressure and surface-deformation monitoring data: Concept and demonstration. Adv. Water Resour. 2013, 62, 555–569. [Google Scholar] [CrossRef]
- Mojtaba, S.; Behzad, R.; Rasoul, N.M.; Mohammad, R. Experimental study of density-driven convection effects on CO2 dissolution rate in formation water for geological storage. J. Nat. Gas Sci. Eng. 2014, 21, 600–607. [Google Scholar] [CrossRef]
- Chen, C.; Wan, J.; Li, W.; Song, Y. Water contact angles on quartz surfaces under supercritical CO2 sequestration conditions: Experimental and molecular dynamics simulation studies. Int. J. Greenh. Gas Control 2015, 42, 655–665. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Kuang, Y.; Fan, Z.; Zhao, Y.; Zhao, J. Influence of core scale permeability on gas production from methane hydrate by thermal stimulation. Int. J. Heat Mass Transf. 2018, 121, 207–214. [Google Scholar] [CrossRef]
- Lu, C.; Lichtner, P.C. High resolution numerical investigation on the effect of convective instability on long term CO2 storage in saline aquifers. In Proceedings of the Scidac 2007: Scientific Discovery through Advanced Computing, Boston, MA, USA, 24–28 June 2007; Volume 78. [Google Scholar]
- Zheng, J.N.; Jiang, L.; Wang, P.; Zhou, H.; Yang, M. MRI observation of CO2-C3H8 hydrate-induced water migration in glass sand. Chem. Eng. Sci. 2019, 207, 1096–1106. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, H.; Wang, P.; Song, Y. Effects of additives on continuous hydrate-based flue gas separation. Appl. Energy 2018, 221, 374–385. [Google Scholar] [CrossRef]
- Borodulin, V.I.; Ivanov, A.V.; Kachanov, Y.S.; Komarova, V.Y. Distributed two-dimensional boundary-layer receptivity to non-stationary vortical disturbances in the presence of surface roughness. Thermophys. Aeromech. 2006, 13, 183–208. [Google Scholar] [CrossRef]
- Zheng, J.N.; Yang, M. Phase Equilibrium Data of CO2-MCP Hydrates and CO2 Gas Uptake Comparisons with CO2-CP Hydrates and CO2-C3H8 Hydrates. J. Chem. Eng. Data 2019, 64, 372–379. [Google Scholar] [CrossRef]
- Ennis-King, J.; Preston, I.; Paterson, L. Onset of convection in anisotropic porous media subject to a rapid change in boundary conditions. Phys. Fluids 2005, 17, 084107. [Google Scholar] [CrossRef]
- Riaz, A.; Hesse, M.; Tchelepi, H.A.; Orr, F.M. Onset of convection in a gravitationally unstable diffusive boundary layer in porous media. J. Fluid Mech. 2006, 548, 87–111. [Google Scholar] [CrossRef]
- Pau, G.S.H.; Bell, J.B.; Pruess, K.; Almgren, A.S.; Lijewski, M.J.; Zhang, K. High-resolution simulation and characterization of density-driven flow in CO storage in saline aquifers. Adv. Water Resour. 2010, 33, 443–455. [Google Scholar] [CrossRef]
- Slim, A.C.; Ramakrishnan, T.S. Onset and cessation of time-dependent, dissolution-driven convection in porous media. Phys. Fluids 2010, 22, 124103. [Google Scholar] [CrossRef]
- Hallworth, M.A.; Huppert, H.E.; Woods, A.W. Dissolution-driven convection in a reactive porous medium. J. Fluid Mech. 2005, 535, 255–285. [Google Scholar] [CrossRef] [Green Version]
- Heidorn, S.C.; Bertram, C.; Cabrera-Sanfelix, P.; Morgenstern, K. Consecutive Mechanism in the Diffusion of D2O on a NaCl (100) Bilayer. ACS Nano 2015, 9, 3572–3578. [Google Scholar] [CrossRef]
- Lu, G.; Liu, Y.; Jiang, L.; Ying, T.; Song, Y.; Wu, B. Study of Density Driven Convection in a Hele-Shaw Cell with Application to the Carbon Sequestration in Aquifers. Energy Procedia 2017, 114, 4303–4312. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, P.; Liu, Y.; Jiang, L.; Tetsuya, S.; Song, Y.; Wu, B.; Liu, S. CO2/water two-phase flow in a two-dimensional micromodel of heterogeneous pores and throats. RSC Adv. 2016, 6, 73897–73905. [Google Scholar] [CrossRef]
- Huppert, H.E.; Neufeld, J.A. The Fluid Mechanics of Carbon Dioxide Sequestration. In Annual Review of Fluid Mechanics; Davis, S.H., Moin, P., Eds.; Annual Reviews: Palo Alto, CA, USA, 2014; Volume 46, pp. 255–272. [Google Scholar]
- Kneafsey, T.J.; Pruess, K. Laboratory Flow Experiments for Visualizing Carbon Dioxide-Induced, Density-Driven Brine Convection. Transp. Porous Media 2010, 82, 123–139. [Google Scholar] [CrossRef] [Green Version]
- Blair, L.M.; Quinn, J.A. The onset of cellular convection in a fluid layer with time- dependent density gradients. J. Fluid Mech. 1969, 36, 385–400. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Hyodo, A.; Wang, L.; Suekane, T. Experimental study of 3D Rayleigh-Taylor convection between miscible fluids in a porous medium. Adv. Water Resour. 2016, 97, 224–232. [Google Scholar] [CrossRef]
- Wang, L.; Hyodo, A.; Sakai, S.; Suekane, T. Three-Dimensional Visualization of Natural Convection in Porous Media. In Proceedings of the 8th Trondheim Conference on CO2 Capture, Transport and Storage, Trondheim, Norway, 16–18 June 2015; Aarlien, R., Rokke, N.A., Svendsen, H.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 86, pp. 460–468. [Google Scholar]
- Cheng, P.; Bestehorn, M.; Firoozabadi, A. Effect of permeability anisotropy on buoyancy-driven flow for CO2 sequestration in saline aquifers. Water Resour. Res. 2012, 48. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Song, Y.; Liu, Y.; Liang, H.; Dou, B. Visualization and Measurement of CO2 Flooding in Porous Media Using MRI. Ind. Eng. Chem. Res. 2011, 50, 4707–4715. [Google Scholar] [CrossRef]
- Zhang, Y.; Sahinidis, N.V. Uncertainty Quantification in CO2 Sequestration Using Surrogate Models from Polynomial Chaos Expansion. Ind. Eng. Chem. Res. 2013, 52, 3121–3132. [Google Scholar] [CrossRef] [Green Version]
- Green, L.L.; Foster, T.D. Secondary convection in a hele shaw cell. J. Fluid Mech. 1975, 71, 675–687. [Google Scholar] [CrossRef]
- Jiang, L.; Wu, B.; Song, Y.; Yang, M.; Wang, D.; Liu, Y.; Xue, Z. Mass transfer coefficient measurement during brine flush in a CO2-filled packed bed by X-ray CT scanning. Int. J. Heat Mass Transf. 2017, 115, 615–624. [Google Scholar] [CrossRef]
- Teng, Y.; Jiang, L.; Fan, Y.; Liu, Y.; Wang, D.; Abudula, A.; Song, Y. Quantifying the dynamic density driven convection in high permeability packed beds. Magn. Reson. Imaging 2017, 39, 168–174. [Google Scholar] [CrossRef]
- Scott, B.; Konstantin, T.; Ecke, R.E. Convective instability and mass transport of diffusion layers in a Hele-Shaw geometry. Phys. Rev. Lett. 2011, 106, 104501. [Google Scholar]
- Sheldon, H.A.; Florio, B.; Trefry, M.G.; Reid, L.B.; Ricard, L.P.; Ghori, K.A.R. The potential for convection and implications for geothermal energy in the Perth Basin, Western Australia. Hydrogeol. J. 2012, 20, 1251–1268. [Google Scholar] [CrossRef]
- Hassanzadeh, H.; Pooladi-Darvish, M.; Keith, D.W. Stability of a fluid in a horizontal saturated porous layer: Effect of non-linear concentration profile, initial, and boundary conditions. Transp. Porous Media 2006, 65, 193–211. [Google Scholar] [CrossRef]
- Teng, Y.; Wang, P.; Jiang, L.; Liu, Y.; Song, Y.; Wei, Y. An experimental study of density-driven convection of fluid pairs with viscosity contrast in porous media. Int. J. Heat Mass Transf. 2020, 152, 119514. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, S.; Abudula, A.; Liu, Y.; Song, Y. The effect of density difference on the development of density-driven convection under large Rayleigh number. Int. J. Heat Mass Transf. 2019, 139, 1087–1095. [Google Scholar] [CrossRef]
- Meybodi, H.E. Comments on the Paper “Quantification of Density-Driven Natural Convection for Dissolution Mechanism in CO2 Sequestration” by R. Nazari Moghaddam et al. (2011). Transp. Porous Media 2012, 93, 171–174. [Google Scholar] [CrossRef]
- Bahar, M.M.; Liu, K. Measurement of The Diffusion Coefficient of CO2 in Formation Water under Reservoir Conditions: Implications for CO2 Storage. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Perth, Australia, 20–22 October 2008; Volume 390, pp. 91–94. [Google Scholar]











| Test No. | Temperature (K) | H (cm) | Salinity (%) | Δρ (kg/m3) | Viscosity (10−3 Pa·s) | Ra |
|---|---|---|---|---|---|---|
| 1 | 306.15 | 20 | 0.00% | 1.001 | 1.04 | 6.561 × 104 |
| 2 | 0.25% | 1.003 | 1.04 | 6.573 × 104 | ||
| 3 | 0.50% | 1.005 | 1.035 | 6.617 × 104 | ||
| 4 | 1.00% | 1.008 | 1.03 | 6.674 × 104 | ||
| 5 | 2.00% | 1.014 | 1.01 | 6.844 × 104 | ||
| 6 | 311.15 | 0.00% | 0.999 | 1.04 | 6.548 × 104 | |
| 7 | 0.25% | 1.001 | 1.04 | 6.560 × 104 | ||
| 8 | 0.50% | 1.003 | 1.035 | 6.605 × 104 | ||
| 9 | 1.00% | 1.007 | 1.03 | 6.662 × 104 | ||
| 10 | 2.00% | 1.012 | 1.01 | 6.832 × 104 | ||
| 11 | 316.15 | 0.00% | 0.997 | 1.04 | 6.536 × 104 | |
| 12 | 0.25% | 0.999 | 1.04 | 6.548 × 104 | ||
| 13 | 0.50% | 1.001 | 1.035 | 6.592 × 104 | ||
| 14 | 1.00% | 1.005 | 1.03 | 6.649 × 104 | ||
| 15 | 2.00% | 1.028 | 1.01 | 6.940 × 104 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Wang, S.; Liu, D.; Zhang, W.; Lu, G.; Liu, Y.; Zhao, J. Change in Convection Mixing Properties with Salinity and Temperature: CO2 Storage Application. Polymers 2020, 12, 2084. https://doi.org/10.3390/polym12092084
Jiang L, Wang S, Liu D, Zhang W, Lu G, Liu Y, Zhao J. Change in Convection Mixing Properties with Salinity and Temperature: CO2 Storage Application. Polymers. 2020; 12(9):2084. https://doi.org/10.3390/polym12092084
Chicago/Turabian StyleJiang, Lanlan, Sijia Wang, Donglei Liu, Weixin Zhang, Guohuan Lu, Yu Liu, and Jiafei Zhao. 2020. "Change in Convection Mixing Properties with Salinity and Temperature: CO2 Storage Application" Polymers 12, no. 9: 2084. https://doi.org/10.3390/polym12092084
APA StyleJiang, L., Wang, S., Liu, D., Zhang, W., Lu, G., Liu, Y., & Zhao, J. (2020). Change in Convection Mixing Properties with Salinity and Temperature: CO2 Storage Application. Polymers, 12(9), 2084. https://doi.org/10.3390/polym12092084