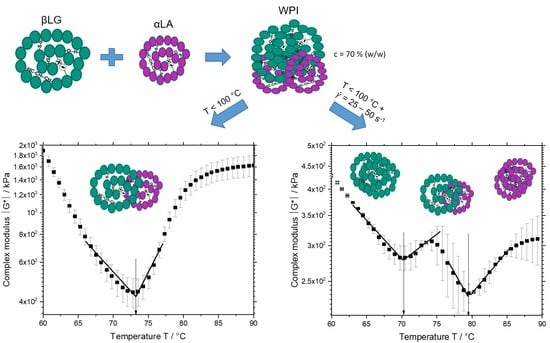
3.1. Influence of Defined Thermal and Mechanical Treatment on the Reaction Behavior and Onset Temperatures of Whey Proteins
The complex modulus
is depicted in
Figure 1 as a function of temperature and shear rate for the system containing 70% (
w/w) WPI. The complex modulus in
Figure 1a shows two distinct regions: initial decrease (Region I) followed by an increase (Region II). The decrease in the complex modulus in Region I was related to the increased mobility of the protein molecules due to increasing temperature. A distinct change in the slope happened at approximately 73 °C (transition from Region I and II), which is defined as the aggregation onset temperature [
9,
10]. Although the aggregation might have started at lower temperatures, this is the temperature where the aggregation outweighs the effect seen in Region I. By increasing the shear rate to 25 and 50 s
−1, the course of the complex modulus changed compared to
Figure 1a. The complex modulus in
Figure 1b,c shows four distinct regions: initial decrease (Region I), followed by an increase (Region II), a further decrease (Region III), and final increase (Region IV), which led to the determination of two aggregation onset temperatures.
At a shear rate of 25 s−1, the aggregation onsets at 70 and 79 °C. By increasing the shear rate to 50 s−1, these temperatures decreased to 66 and 75 °C. The occurrence of two onset temperatures when the samples were sheared might suggest shear induced phase separation of proteins. At a shear rate of 0.06 s−1, the protein molecules of different fractions (i.e., βLG and αLA) were homogenously distributed, and the measured onset temperature implied that they involve in the aggregation reaction simultaneously. At higher shear rates, it is possible that these protein fractions form separated phases due to their limited miscibility, which might lead to variations of the molecular mobility, interactions, and aggregation at the local level. This possibility is discussed in the following sections in more detail.
The results for βLG and αLA samples depicted in
Figure 2a and
Figure 3a show that there were two temperatures at which the course of the complex modulus changed. These temperatures are defined as the denaturation and aggregation onset temperature, respectively. As shown in
Figure 2a, the denaturation onset temperature for βLG with a concentration of 70% was approximately 72 °C, which is in accordance with previous results in [
9]. In the case of αLA (
Figure 3a), a lower denaturation of approximately 65 °C was measured. For both proteins, increasing the shear rate (depicted in b, c) led to a decrease in the aggregation onset temperature.
In contrast to WPI, which is a complex system containing more than one protein fraction, the aggregation onset temperature at a shear rate of 0.06 s
−1 for systems containing the single fractions αLA and βLG increased from 73 to 82 °C and 80 °C, respectively. As expected, αLA shows higher aggregation onset temperatures compared to βLG due to its four stabilizing disulfide bonds and lacking a free thiol group. Higher aggregation temperatures were observed for αLA compared to βLG from various authors at various treatment conditions [
11,
12]. The results of WPI at 0.06 s
−1 show that the βLG had a lower aggregation temperature in this complex system. Since the WPI systems show lower viscosity as the βLG systems, higher reaction rates were expected due to an increased molecular motion, which explained the lower onset temperature. Additionally, an increase in reaction rate was expected as in WPI systems all protein fractions present could participate and form aggregates. Although no denaturation onset temperature was observed for the WPI at a shear rate of 0.06 s
−1, it was expected that denaturation onsets between 60 and 70 °C.
In accordance to these results, [
13] showed that the denaturation onset temperature of βLG decreased when βLG was combined with αLA. The authors also showed that systems containing only αLA showed a denaturation temperature of approximately 65 °C. Since αLA denatured at lower temperatures compared to βLG, it seems possible that in homogenous systems containing both fractions, the aggregation reactions starts from a reactive αLA monomer if the thermal treatment is high enough to activate the αLA monomers. Furthermore, the concentration of each fraction present in the mixture was also expected to influence denaturation and aggregation reactions.
At a shear rate of 25 s
−1, βLG shows an aggregation onset temperature of 70 °C and αLA of 79 °C. Increasing the shear rate to 50 s
−1 led to a decrease in the aggregation onset temperature to 66 and 76 °C, for βLG and αLA, respectively. This effect was also observed in our previous studies on WPI and βLG [
7,
10]. It seems that the aggregation onset temperatures determined for the sheared WPI systems corresponded to the onset temperatures of the single protein fractions. This can be related to the phase separation phenomena, which has been observed for various biopolymer mixtures [
14]. For instance, [
15] reported for βLG and κ-carrageenan (κ-Car) systems two slopes of the elastic modulus G’ during a heating/shearing and cooling down treatment step. The first slope corresponded to the setting of the βLG network, and the second to the further protein network affected by the formation of the κ-Car gel in the system [
16]. Although the authors investigated a mixture of globular proteins and polysaccharide (i.e., βLG and κ-car), similar to the results observed in
Figure 1 during heating and shearing of WPI systems, two markedly different rheological behaviors (i.e., a bicontinuous profile) were observed suggesting the formation of phase-separated gels. Similarly, the first onset temperature suggests the setting of βLG aggregates and the second one, the aggregation of αLA affecting the previously formed βLG network.
Although phase separation of protein solutions has not been often reported, it is indeed expected to occur with time [
17]. Since proteins have, like all polymers or all solid materials, a limited solubility, at a protein concentration above their solubility, separation into different phases occur [
18].
3.2. Influence of Defined Thermal and Mechanical Treatment on the Denaturation of Whey Proteins
The influence of treatment temperature and shear rate on the
DD for samples containing WPI and αLA at a concentration of 70% (
w/w) treated for 30 s is depicted in
Figure 4 and
Figure 5, respectively.
As shown in
Figure 4, thermal treatment of WPI at 60 and 70 °C resulted in a degree of denaturation of 2% and 12%, respectively. The degree of denaturation for samples treated thermally at 80 °C was approximately 80%. At higher temperatures, the degree of denaturation was already above 90% even at the lower shear rate of 0.06 s
−1. Increasing the shear rate to 50 s
−1 led to an increase in denaturation for all temperatures investigated. Thermomechanical treatment of WPI at a shear rate of 50 s
−1 and temperatures 60, 70, and 80 °C resulted in a degree of denaturation of approximately 13%, 51%, and 94%, respectively. At temperatures above 100 °C, the effect of the shear rate on the denaturation was no longer visible as at these conditions the denaturation reaction was already high.
As shown in [
7], the thermal treatment of βLG at a concentration of 70% resulted in similar denaturation compared to WPI. Treatment of βLG systems at 60, 70, and 80 °C resulted in a degree of denaturation of approximately 2%, 11%, and 72%, respectively, whereas thermomechanical treatment at a shear rate of 50 s
−1 and temperatures 60, 70, and 80 °C resulted in a degree of denaturation of approximately 22%, 58%, and 96%, respectively.
Although the denaturation of βLG and WPI systems at a shear rate of 0.06 s
−1 was very similar, at higher shear rates the βLG systems showed higher degrees of denaturation. Probably due to the fact that the βLG systems show higher values of complex modulus and by this, of viscosity (
Figure 1 and
Figure 2), therefore the effect of the shear stresses on the reactions is expected to increase, which could then lead to higher reaction rates.
As depicted in
Figure 5, the degree of denaturation of αLA was higher compared to WPI and βLG independent of the applied treatment temperature or shear rate. At a shear rate of 0.06 s
−1, treatment at temperatures of 60, 70, and 80 °C resulted in approximately 7%, 52%, and 87%, respectively. At a shear rate of 50 s
−1, even a treatment at 60 °C resulted in approximately 40% denaturation. Samples treated at 70 °C and 50 s
−1 were over 80% denatured. Higher treatment temperatures resulted in almost a complete denaturation since the degree of denaturation was above 80% even at the lowest shear rate of 0.06 s
−1.
Although the denaturation temperature for aLA solutions determined using differential scanning calorimetry (DSC) was approximately 65 °C [
11,
13,
19,
20,
21], when αLA was heated at defined temperatures (below 100 °C), reversible denaturation through the formation of non-covalent bonds was observed. Due to the weak nature of these bonds, the newly formed bonds break up as soon as samples cool down, αLA returns to a quasi-native state, and renaturation takes place [
22].
The overall high values of denaturation observed in
Figure 5 could result either from the high concentration of αLA or from the small amount of βLG (approximately 5%) present in the αLA protein powder. If the reaction rate is high enough, which is the case at high protein concentrations, then the intermolecular thiol-disulfide exchange is rapid and the probability of renaturation decreases [
11], leading to high values of denaturation. Additionally it is possible, that the small amount of βLG present in the αLA protein powder could act as a catalyst for the reactions, making a protein molecule available with a free thiol group, which then could react with an αLA molecule, initiating the aggregation reaction.
3.3. Kinetics of Denaturation of Whey Proteins at Extrusion-Like Conditions
Data from the results of the degree of denaturation was plotted and fitted using NLR to obtain the kinetic parameters shown in
Table 1 and
Table 2.
The kinetic parameters for the denaturation reaction for αLA after thermal and mechanical treatment at a concentration of 70% (
w/w) are shown in
Table 1. For the investigated range of treatment conditions, a denaturation reaction order of 2.262 was identified. The reaction rate constant (
k) increased with temperature and a combination of thermal and mechanical treatment led to even higher values of
k. The reaction rate determined in [
7] for βLG at concentrations between 50% and 70% was 2.151, which is very similar to the reaction rate shown in
Table 1.
In contrast, the reaction order for WPI at a concentration of 60% was 1.909 [
10]. A similar reaction order (1.865) was determined when the concentration of the WPI system increased to 70%. Although compared to our previous results the denaturation reaction order decreased slightly from 1.909 to 1.865 when the concentration of WPI increased from 60% to 70%, the accelerating effect of temperature and shear rate on the reactions was still observed. There is clearly a dependence of protein fractions present in the protein system on the reaction order. The reaction order for single protein fractions follows a fractional second order, whereas for complex systems such as WPI where multiple protein fractions are present, a fractional first order was observed. In theory, a second order is observed when the reactions depend on the concentration of two reactants or one concentration squared. For βLG it was hypothesized that a reactive monomer is needed to start the reaction, consequently starting from dimers, then native monomers are formed, which then are activated and react further with other monomers to form non-native dimers, trimers, and eventually oligomers [
23]. Consequently, the denaturation reaction depends on the concentrations of dimers and reactive monomers available. Denaturation of αLA is expected to follow a similar mechanism, starting from monomers, which need a monomer with a free thiol group to react. When the thermal treatment is high enough the disulfide bonds stabilizing the αLA molecule are broken down. As shown in [
12], thermal treatment at 100 °C for 10–30 min results in up to a 25% loss of disulfide bonds, which could lead to the formation of reactive groups similar to thiols. Similar to βLG, the reactive αLA monomers react then with monomers and form disulfide bonded oligomers through thiol-disulfide exchange reactions. For the WPI systems, either the free thiol group of the βLG can react with βLG monomers or αLA monomers, therefore the reactions depend mostly on the concentration of the thiol groups available resulting in a fractional first order. Although for WPI a first fractional order reaction was observed, the reaction order is higher than most of the findings of previous studies for lower protein concentrations at neutral pH. Most of the kinetic data for the denaturation reaction of whey proteins takes only into consideration the thermal denaturation of βLG in the system and a reaction order of 1.5 is frequently observed. The increase in the reaction order at the investigated conditions could arise either from the increased protein concentration or from the additional effect of shear stresses on the reactions. As shown in
Figure 1, for systems containing multiple protein fractions, a combination of thermal and mechanical treatment led to a more complex reaction behavior as two aggregation onset temperatures were observed. The reaction order of almost 2 (1.865 and 1.909) could then result from a combination of a lower reaction order for unsheared systems and higher reaction order for sheared systems as phase separation occurs, leading to similar reaction orders as for the single protein fractions. Consequently, it is expected that the denaturation reaction rate after thermomechanical treatment of complex systems containing various protein fractions depends on the ratio and concentration of each fraction. As already shown for less concentrated systems, changes of βLG and αLA ratios in WPI systems result in aggregates with different protein composition [
24].
The calculated values of the activation energy (
Et) for αLA and WPI systems at a concentration of 70% are depicted in
Table 2. The results show that the activation energy decreased with increasing thermal and mechanical treatment. Similarly results are shown in [
7] for βLG systems with a concentration of 70%. As expected from the previously discussed results, in the temperature range of 80–100 °C the activation energy was highest for βLG (107–133 kJ mol
−1) [
7], then for αLA (109–117 kJ mol
−1) followed by WPI (80–100 kJ mol
−1). Since at temperatures above 80 °C both of the protein fractions (αLA and βLG) are expected to aggregate, the energy input in the system due to the treatment was high enough to induce aggregation, consequently the activation energy was lowest for WPI systems. In the temperature range between 60 and 80 °C, the activation energy was lowest for the αLA systems. Since αLA denatured at approximately 65 °C, treatment in this temperature range positively affected the reaction leading to lower values of activation energies. Similar results were observed in [
25] since the denaturation of αLA in solutions was found to be initially even faster than that of βLG at 65 °C. The maximum reduction of the activation energy at a shear rate of 50 s
−1 was 19% and 12% for αLA and WPI, respectively. In WPI systems, the intermolecular interactions between αLA and βLG possibly resulting in phase separation, led to higher values of activation energy and the effect on the shear rate on the reduction of activation energy seemed to decrease. Furthermore, for αLA systems by increasing the shear rate from 25 to 50 s
−1, the activation energy decreased by only 1%, which could imply that shear stress indeed influences the reactions, but only up to a limit and the maximum reduction is achieved at the chosen shear rates. The activation energy determined for the βLG systems was overall higher than for αLA and WPI. As already shown in the results from temperature sweeps, the complex modulus of the βLG systems was higher than for αLA and WPI systems. The high complex modulus and by this high viscosity led to low molecular mobility, lower reaction rates, and higher values of activation energy. Therefore, the calculated activation energies for WPI systems and the effect of the shear rate on its reduction represent the combined effect observed for the main fractions, βLG and αLA. Similar results were observed by [
26], since the denaturation rate for βLG was lower than for systems containing more than one whey protein fraction. Overall, the determined activation energies are in agreement with previously reported values by other authors [
26,
27]. In the temperature range below and above 80 °C, the activation energy values for various whey protein systems ranged from 189 to 283 kJ mol
−1 and between 80 and 133 kJ mol
−1, respectively.