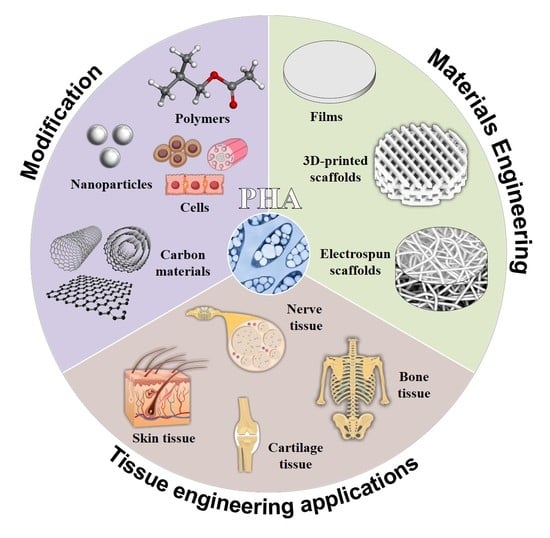
Review of Hybrid Materials Based on Polyhydroxyalkanoates for Tissue Engineering Applications
Abstract
:1. Introduction
2. The Most Important Properties of the Hybrids Based on PHAs
2.1. Wettability of the Composites
2.2. Physico-Mechanical Properties
2.3. Biodegradation of the Hybrids
2.4. Piezoelectric Properties
3. PHA Based Composites for Tissue Engineering
3.1. Bone Tissue Engineering
- Mechanical strength to withstand hydrostatic pressure.
- Osteoinductivity to promote the migration of osteogenic cells and stimulate differentiation. An important role in osteoinductivity is played by the chemical composition of the scaffold, its porosity, surface properties and nano/microtopography.
- Porosity to provide delivery of nutrients to cells, remove cellular waste and promote vascularization. Fabrication of porous biocompatible PHA-based materials makes them more suitable for cell growth and allows cells to penetrate into the scaffold. Pore size should be at least 100 μm in diameter for successful diffusion of essential nutrients and oxygen supply. However, pore sizes in the range of 200 to 350 μm were found to be optimal for bone tissue in-growth [9,123].
- Vascularization to avoid ischaemia and cell apoptosis.
- Bioresorbability to allow new bone tissue formation. The scaffolds should degrade at a controlled resorption rate, creating space for new bone tissue formation. Degradation products should not cause inflammation to the surrounding tissues.
3.2. Cartilage Tissue Engineering
3.3. Nerve Tissue Engineering
3.4. Skin Tissue Regeneration and Wound Healing
4. Future Prospects and Challenges
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Favaro, L.; Basaglia, M.; Casella, S. Improving polyhydroxyalkanoate production from inexpensive carbon sources by genetic approaches: A review. Biofuels Bioprod. Biorefining 2019, 13, 208–227. [Google Scholar] [CrossRef] [Green Version]
- Saratale, R.G.; Cho, S.-K.; Saratale, G.D.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Mulla, S.I. A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour. Technol. 2021, 325, 124685. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Wang, Y.; Tong, Y.; Chen, G.-Q. Grand Challenges for Industrializing Polyhydroxyalkanoates (PHAs). Trends Biotechnol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Akaraonye, E.; Keshavarz, T.; Roy, I. Production of polyhydroxyalkanoates: The future green materials of choice. J. Chem. Technol. Biotechnol. 2010, 85, 732–743. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Cho, S.K.; Kim, D.S.; Ghodake, G.S.; Kadam, A.; Kumar, G.; Bharagava, R.N.; Banu, R.; Shin, H.S. Pretreatment of kenaf (Hibiscus cannabinus L.) biomass feedstock for polyhydroxybutyrate (PHB) production and characterization. Bioresour. Technol. 2019, 282, 75–80. [Google Scholar] [CrossRef]
- Sirohi, R.; Pandey, J.P.; Gaur, V.K.; Gnansounou, E.; Sindhu, R. Critical overview of biomass feedstocks as sustainable substrates for the production of polyhydroxybutyrate (PHB). Bioresour. Technol. 2020, 311, 123536. [Google Scholar] [CrossRef]
- Hazer, D.B.; Kılıçay, E.; Hazer, B. Poly(3-hydroxyalkanoate)s: Diversification and biomedical applications: A state of the art review. Mater. Sci. Eng. C 2012, 32, 637–647. [Google Scholar] [CrossRef]
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [Green Version]
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174. [Google Scholar] [CrossRef]
- Zine, R.; Sinha, M. Nanofibrous poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/collagen/graphene oxide scaffolds for wound coverage. Mater. Sci. Eng. C 2017, 80, 129–134. [Google Scholar] [CrossRef]
- Yang, H.-X.; Sun, M.; Zhang, Y.; Zhou, P. Degradable PHBHHx modified by the silk fibroin for the applications of cardiovascular tissue engineering. ISRN Mater. Sci. 2011, 2011, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Heidari-Keshel, S.; Ahmadian, M.; Biazar, E.; Gazmeh, A.; Rabiei, M.; Adibi, M.; Shabani, M. Surface modification of Poly Hydroxybutyrate (PHB) nanofibrous mat by collagen protein and its cellular study. Mater. Technol. 2016, 31, 799–805. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Vatankhah, E.; Ramakrishna, S. Electrospun aligned PHBV/collagen nanofibers as substrates for nerve tissue engineering. Biotechnol. Bioeng. 2013, 110, 2775–2784. [Google Scholar] [CrossRef]
- Chiulan, I.; Mihaela Panaitescu, D.; Nicoleta Frone, A.; Teodorescu, M.; Andi Nicolae, C.; Căşărică, A.; Sălăgeanu, A. Biocompatible polyhydroxyalkanoates/bacterial cellulose composites: Preparation, characterization, and in vitro evaluation. J. Biomed. Mater. Res. Part A 2016, 104, 2576–2584. [Google Scholar] [CrossRef]
- Zarei, M.; Karbasi, S. Evaluation of the effects of multiwalled carbon nanotubes on electrospun poly (3-hydroxybutirate) scaffold for tissue engineering applications. J. Porous Mater. 2018, 25, 259–272. [Google Scholar] [CrossRef]
- Tesema, Y.; Raghavan, D.; Stubbs, J., III. Bone cell viability on collagen immobilized poly (3-hydroxybutrate-co-3-hydroxyvalerate) membrane: Effect of surface chemistry. J. Appl. Polym. Sci. 2004, 93, 2445–2453. [Google Scholar] [CrossRef]
- Andreessen, B.; Taylor, N.; Steinbüchel, A. Poly (3-hydroxypropionate): A promising alternative to fossil fuel-based materials. Appl. Environ. Microbiol. 2014, 80, 6574–6582. [Google Scholar] [CrossRef] [Green Version]
- Aeschelmann, F.; Carus, M. Biobased building blocks and polymers in the world: Capacities, production, and applications—Status quo and trends towards 2020. Ind. Biotechnol. 2015, 11, 154–159. [Google Scholar] [CrossRef]
- Market, M.A. Polyhydroxyalkanoate (PHA) Market by Type (Short Chain Length, Medium Chain Length), Production Method (Sugar Fermentation, Vegetable Oil Fermentation, Methane Fermentation), Application, and Region-Global Forecast to 2024. Markets and Markets Research Private Ltd., 2019. Available online: https://www.prnewswire.com/ (accessed on 18 December 2017).
- Saratale, G.D.; Bhosale, R.; Pugazendhi, A.; Mahmoud, E.; Sirohi, R.; Bhatia, S.K.; Shin, H.S. A review on valorization of spent coffee grounds (SCG) towards biopolymers and biocatalysts production. Bioresour. Technol. 2020, 314, 123800. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Varjani, S.; Cho, S.-K.; Ghodake, G.S.; Kadam, A. Development of ultrasound aided chemical pretreatment methods to enrich saccharification of wheat waste biomass for polyhydroxybutyrate production and its characterization. Ind. Crop. Prod. 2020, 150, 112425. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.-K.; Ghodake, G.S.; Shin, H.-S.; Saratale, G.D.; Park, Y.; Lee, H.-S.; Bharagava, R.N.; Kim, D.-S. Utilization of noxious weed water hyacinth biomass as a potential feedstock for biopolymers production: A novel approach. Polymers 2020, 12, 1704. [Google Scholar] [CrossRef]
- Sultana, N.; Khan, T.H. In vitro degradation of PHBV scaffolds and nHA/PHBV composite scaffolds containing hydroxyapatite nanoparticles for bone tissue engineering. J. Nanomater. 2012, 2012, 1–12. [Google Scholar]
- Kumarasuriyar, A.; Jackson, R.A.; Grøndahl, L.; Trau, M.; Nurcombe, V.; Cool, S.M. Poly (β-hydroxybutyrate-co-β-hydroxyvalerate) supports in vitro osteogenesis. Tissue Eng. 2005, 11, 1281–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Gao, R.; Wang, P.-P.; Jian, J.; Jiang, X.-L.; Yan, C.; Lin, X.; Wu, L.; Chen, G.-Q.; Wu, Q. The differential effects of aligned electrospun PHBHHx fibers on adipogenic and osteogenic potential of MSCs through the regulation of PPARγ signaling. Biomaterials 2012, 33, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bian, Y.-Z.; Wu, Q.; Chen, G.-Q. Evaluation of three-dimensional scaffolds prepared from poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) for growth of allogeneic chondrocytes for cartilage repair in rabbits. Biomaterials 2008, 29, 2858–2868. [Google Scholar] [CrossRef] [PubMed]
- Goonoo, N.; Bhaw-Luximon, A.; Passanha, P.; Esteves, S.R.; Jhurry, D. Third generation poly (hydroxyacid) composite scaffolds for tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1667–1684. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wu, Q.; Yang, F.; Xu, M.; Leski, M.; Chen, G.-Q. Influence of DL-β-hydroxybutyric acid on cell proliferation and calcium influx. Biomacromolecules 2005, 6, 593–597. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Wu, Q.; Chen, G.-Q. Attachment, proliferation and differentiation of osteoblasts on random biopolyester poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds. Biomaterials 2004, 25, 669–675. [Google Scholar] [CrossRef]
- Ang, S.L.; Shaharuddin, B.; Chuah, J.-A.; Sudesh, K. Electrospun poly (3-hydroxybutyrate-co-3-hydroxyhexanoate)/silk fibroin film is a promising scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 145, 173–188. [Google Scholar] [CrossRef]
- Sadeghi, D.; Karbasi, S.; Razavi, S.; Mohammadi, S.; Shokrgozar, M.A.; Bonakdar, S. Electrospun poly (hydroxybutyrate)/chitosan blend fibrous scaffolds for cartilage tissue engineering. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Shishatskaya, E.I.; Khlusov, I.A.; Volova, T.G. A hybrid PHB–hydroxyapatite composite for biomedical application: Production, in vitro and in vivo investigation. J. Biomater. Sci. Polym. Ed. 2006, 17, 481–498. [Google Scholar] [CrossRef]
- Ding, Y.; Roether, J.A.; Boccaccini, A.R.; Schubert, D.W. Fabrication of electrospun poly (3-hydroxybutyrate)/poly (ε-caprolactone)/silica hybrid fibermats with and without calcium addition. Eur. Polym. J. 2014, 55, 222–234. [Google Scholar] [CrossRef]
- Misra, S.K.; Mohn, D.; Brunner, T.J.; Stark, W.J.; Philip, S.E.; Roy, I.; Salih, V.; Knowles, J.C.; Boccaccini, A.R. Comparison of nanoscale and microscale bioactive glass on the properties of P (3HB)/Bioglass® composites. Biomaterials 2008, 29, 1750–1761. [Google Scholar] [CrossRef]
- Sabapathy, P.C.; Devaraj, S.; Meixner, K.; Anburajan, P.; Kathirvel, P.; Ravikumar, Y.; Qi, X. Recent developments in Polyhydroxyalkanoates (PHAs) production—A review. Bioresour. Technol. 2020, 306, 123132. [Google Scholar] [CrossRef]
- Pagliano, G.; Galletti, P.; Samorì, C.; Zaghini, A.; Torri, C. Recovery of Polyhydroxyalkanoates From Single and Mixed Microbial Cultures: A Review. Front. Bioeng. Biotechnol. 2021, 9, 54. [Google Scholar] [CrossRef]
- Giubilini, A.; Bondioli, F.; Messori, M.; Nyström, G.; Siqueira, G. Advantages of Additive Manufacturing for Biomedical Applications of Polyhydroxyalkanoates. Bioengineering 2021, 8, 29. [Google Scholar] [CrossRef]
- Tarrahi, R.; Fathi, Z.; Seydibeyoğlu, M.Ö.; Doustkhah, E.; Khataee, A. Polyhydroxyalkanoates (PHA): From production to nanoarchitecture. Int. J. Biol. Macromol. 2020, 146, 596–619. [Google Scholar] [CrossRef]
- Ang, S.L.; Sivashankari, R.; Shaharuddin, B.; Chuah, J.-A.; Tsuge, T.; Abe, H.; Sudesh, K. Potential Applications of Polyhydroxyalkanoates as a Biomaterial for the Aging Population. Polym. Degrad. Stab. 2020, 181, 109371. [Google Scholar] [CrossRef]
- Chuenjitkuntaworn, B.; Inrung, W.; Damrongsri, D.; Mekaapiruk, K.; Supaphol, P.; Pavasant, P. Polycaprolactone/hydroxyapatite composite scaffolds: Preparation, characterization, and in vitro and in vivo biological responses of human primary bone cells. J. Biomed. Mater. Res. Part A 2010, 94, 241–251. [Google Scholar] [CrossRef]
- Guzmán, D.; Kirsebom, H.; Solano, C.; Quillaguamán, J.; Hatti-Kaul, R. Preparation of hydrophilic poly (3-hydroxybutyrate) macroporous scaffolds through enzyme-mediated modifications. J. Bioact. Compat. Polym. 2011, 26, 452–463. [Google Scholar] [CrossRef]
- Köse, G.T.; Korkusuz, F.; Korkusuz, P.; Purali, N.; Özkul, A.; Hasırcı, V. Bone generation on PHBV matrices: An in vitro study. Biomaterials 2003, 24, 4999–5007. [Google Scholar] [CrossRef]
- Wang, N.; Zhou, Z.; Xia, L.; Dai, Y.; Liu, H. Fabrication and characterization of bioactive β-Ca2SiO4/PHBV composite scaffolds. Mater. Sci. Eng. C 2013, 33, 2294–2301. [Google Scholar] [CrossRef] [PubMed]
- Sousa, I.; Mendes, A.; Pereira, R.F.; Bártolo, P.J. Collagen surface modified poly (ε-caprolactone) scaffolds with improved hydrophilicity and cell adhesion properties. Mater. Lett. 2014, 134, 263–267. [Google Scholar] [CrossRef]
- Kim, M.S.; Na Shin, Y.; Cho, M.H.; Kim, S.H.; Kim, S.K.; Cho, Y.H.; Khang, G.; Lee, I.W.; Lee, H.B. Adhesion behavior of human bone marrow stromal cells on differentially wettable polymer surfaces. Tissue Eng. 2007, 13, 2095–2103. [Google Scholar] [CrossRef]
- Lee, J.H.; Khang, G.; Lee, H.B. Interaction of cells on chargeable functional group gradient surfaces. Biomaterials 1997, 18, 351–358. [Google Scholar] [CrossRef]
- Misra, S.K.; Nazhat, S.N.; Valappil, S.P.; Moshrefi-Torbati, M.; Wood, R.J.K.; Roy, I.; Boccaccini, A.R. Fabrication and characterization of biodegradable poly (3-hydroxybutyrate) composite containing bioglass. Biomacromolecules 2007, 8, 2112–2119. [Google Scholar] [CrossRef]
- Ishaug-Riley, S.L.; Okun, L.E.; Prado, G.; Applegate, M.A.; Ratcliffe, A. Human articular chondrocyte adhesion and proliferation on synthetic biodegradable polymer films. Biomaterials 1999, 20, 2245–2256. [Google Scholar] [CrossRef]
- Karahaliloğlu, Z. Cell-compatible PHB/silk fibroin composite nanofiber mat for tissue engineering applications. Turk. J. Biol. 2017, 41, 503–513. [Google Scholar] [CrossRef]
- Lei, C.; Zhu, H.; Li, J.; Li, J.; Feng, X.; Chen, J. Preparation and characterization of polyhydroxybutyrate-co-hydroxyvalerate/silk fibroin nanofibrous scaffolds for skin tissue engineering. Polym. Eng. Sci. 2014, 55, 907–916. [Google Scholar] [CrossRef]
- Karbasi, S.; Karimi, A.; Razavi, S.; Zargar, E.N. Poly (hydroxybutyrate)/chitosan aligned electrospun scaffold as a novel substrate for nerve tissue engineering. Adv. Biomed. Res. 2018, 7, 44. [Google Scholar] [CrossRef]
- Hu, S.-G.; Jou, C.-H.; Yang, M.-C. Biocompatibility and antibacterial activity of chitosan and collagen immobilized poly (3-hydroxybutyric acid-co-3-hydroxyvaleric acid). Carbohydr. Polym. 2004, 58, 173–179. [Google Scholar] [CrossRef]
- Mohammadalizadeh, Z.; Karbasi, S.; Arasteh, S. Physical, mechanical and biological evaluation of poly (3-hydroxybutyrate)-chitosan/MWNTs as a novel electrospun scaffold for cartilage tissue engineering applications. Polym. Plast. Technol. Mater. 2020, 59, 417–429. [Google Scholar] [CrossRef]
- Foroughi, M.R.; Karbasi, S.; Khoroushi, M.; Khademi, A.A. Polyhydroxybutyrate/chitosan/bioglass nanocomposite as a novel electrospun scaffold: Fabrication and characterization. J. Porous Mater. 2017, 24, 1447–1460. [Google Scholar] [CrossRef]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef]
- Ito, Y.; Hasuda, H.; Kamitakahara, M.; Ohtsuki, C.; Tanihara, M.; Kang, I.-K.; Kwon, O.H. A composite of hydroxyapatite with electrospun biodegradable nanofibers as a tissue engineering material. J. Biosci. Bioeng. 2005, 100, 43–49. [Google Scholar] [CrossRef]
- Ramier, J.; Bouderlique, T.; Stoilova, O.; Manolova, N.; Rashkov, I.; Langlois, V. Biocomposite scaffolds based on electrospun poly (3-hydroxybutyrate) nanofibers and electrosprayed hydroxyapatite nanoparticles for bone tissue engineering applications. Mater. Sci. Eng. C 2014, 38, 161–169. [Google Scholar] [CrossRef]
- Wu, T.-J.; Huang, H.-H.; Lan, C.-W.; Lin, C.-H.; Hsu, F.-Y.; Wang, Y.-J. Studies on the microspheres comprised of reconstituted collagen and hydroxyapatite. Biomaterials 2004, 25, 651–658. [Google Scholar] [CrossRef]
- Tachibana, A.; Kaneko, S.; Tanabe, T.; Yamauchi, K. Rapid fabrication of keratin–hydroxyapatite hybrid sponges toward osteoblast cultivation and differentiation. Biomaterials 2005, 26, 297–302. [Google Scholar] [CrossRef]
- Hosoya, K.; Ohtsuki, C.; Kawai, T.; Kamitakahara, M.; Ogata, S.-i.; Miyazaki, T. A novel covalently crosslinked gel of alginate and silane with the ability to form bone-like apatite. J. Biomed. Mater. Res. Part A 2004, 71, 596–601. [Google Scholar] [CrossRef]
- Furuzono, T.; Wang, P.-L.; Korematsu, A.; Miyazaki, K.; Oido-Mori, M.; Kowashi, Y.; Ohura, K.; Tanaka, J.; Kishida, A. Physical and biological evaluations of sintered hydroxyapatite/silicone composite with covalent bonding for a percutaneous implant material. J. Biomed. Mater. Res. Part B 2003, 65, 217–226. [Google Scholar] [CrossRef]
- Chou, Y.-F.; Huang, W.; Dunn, J.C.; A Miller, T.; Wu, B.M. The effect of biomimetic apatite structure on osteoblast viability, proliferation, and gene expression. Biomaterials 2005, 26, 285–295. [Google Scholar] [CrossRef]
- Lin, X.; Yin, M.; Liu, Y.; Li, L.; Ren, X.; Sun, Y.; Huang, T.-S. Biodegradable polyhydroxybutyrate/poly-ε-caprolactone fibrous membranes modified by silica composite hydrol for super hydrophobic and outstanding antibacterial application. J. Ind. Eng. Chem. 2018, 63, 303–311. [Google Scholar] [CrossRef]
- Rivera-Briso, A.L.; Aachmann, F.L.; Moreno-Manzano, V.; Serrano-Aroca, A. Graphene oxide nanosheets versus carbon nanofibers: Enhancement of physical and biological properties of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) films for biomedical applications. Int. J. Biol. Macromol. 2020, 143, 1000–1008. [Google Scholar] [CrossRef]
- Du, Z.; Jia, S.; Xiong, P.; Cai, Z. Preparation of protein nanoparticle-coated poly (hydroxybutyrate) electrospun nanofiber based scaffold for biomedical applications. Int. J. Polym. Mater. Polym. Biomater. 2021, 1–15. [Google Scholar] [CrossRef]
- Zarei, M.; Karbasi, S.; Aslani, F.S.; Zare, S.; Koohi-Hosseinabad, O.; Tanideh, N. In Vitro and In Vivo Evaluation of Poly (3-hydroxybutyrate)/Carbon Nanotubes Electrospun Scaffolds for Periodontal Ligament Tissue Engineering. J. Dent. 2020, 21, 18. [Google Scholar]
- Zhijiang, C.; Cong, Z.; Jie, G.; Qing, Z.; Kongyin, Z. Electrospun carboxyl multi-walled carbon nanotubes grafted polyhydroxybutyrate composite nanofibers membrane scaffolds: Preparation, characterization and cytocompatibility. Mater. Sci. Eng. C 2018, 82, 29–40. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Q.; Cai, Z.; Zhao, K. Preparation and dye filtration property of electrospun polyhydroxybutyrate–calcium alginate/carbon nanotubes composite nanofibrous filtration membrane. Sep. Purif. Technol. 2016, 161, 69–79. [Google Scholar] [CrossRef]
- Ai, J.; Heidari K, S.; Ghorbani, F.; Ejazi, F.; Biazar, E.; Asefnejad, A.; Montazeri, M. Fabrication of coated-collagen electrospun PHBV nanofiber film by plasma method and its cellular study. J. Nanomater. 2011. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, E.D.; Pappas, D.; Güçeri, S.; Sun, W. Enhanced cellular functions on polycaprolactone tissue scaffolds by O2 plasma surface modification. Plasma Process. Polym. 2011, 8, 256–267. [Google Scholar] [CrossRef]
- Domingos, M.; Intranuovo, F.; Gloria, A.; Gristina, R.; Ambrosio, L.; Bártolo, P.J.; Favia, P. Improved osteoblast cell affinity on plasma-modified 3-D extruded PCL scaffolds. Acta Biomater. 2013, 9, 5997–6005. [Google Scholar] [CrossRef]
- Bormashenko, E.; Chaniel, G.; Grynyov, R. Towards understanding hydrophobic recovery of plasma treated polymers: Storing in high polarity liquids suppresses hydrophobic recovery. Appl. Surf. Sci. 2013, 273, 549–553. [Google Scholar] [CrossRef]
- Yang, H.; Sun, M.; Zhou, P.; Pan, L.; Wu, C. Silk fibroins modify the atmospheric low temperature plasma-treated poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) film for the application of cardiovascular tissue engineering. J. Biomed. Sci. Eng. 2010, 3, 1146. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-P.; Su, C.-H. Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatin immobilization for cartilage tissue engineering. Acta Biomater. 2011, 7, 234–243. [Google Scholar] [CrossRef]
- Unalan, I.; Colpankan, O.; Albayrak, A.Z.; Gorgun, C.; Urkmez, A.S. Biocompatibility of plasma-treated poly (3-hydroxybutyrate-co-3-hydroxyvalerate) nanofiber mats modified by silk fibroin for bone tissue regeneration. Mater. Sci. Eng. C 2016, 68, 842–850. [Google Scholar] [CrossRef]
- Zamanifard, M.; Khorasani, M.T.; Daliri, M.; Parvazinia, M. Preparation and modeling of electrospun polyhydroxybutyrate/polyaniline composite scaffold modified by plasma and printed by an inkjet method and its cellular study. J. Biomater. Sci. Polym. Ed. 2020, 31, 1515–1537. [Google Scholar] [CrossRef]
- Reignier, J.; Huneault, M.A. Preparation of interconnected poly (ε-caprolactone) porous scaffolds by a combination of polymer and salt particulate leaching. Polymer 2006, 47, 4703–4717. [Google Scholar] [CrossRef] [Green Version]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Tai, H.-Y.; Fu, E.; Cheng, L.-P.; Don, T.-M. Fabrication of asymmetric membranes from polyhydroxybutyrate and biphasic calcium phosphate/chitosan for guided bone regeneration. J. Polym. Res. 2014, 21, 421. [Google Scholar] [CrossRef]
- Tanideh, N.; Azarpira, N.; Sarafraz, N.; Zare, S.; Rowshanghiyas, A.; Farshidfar, N.; El Fray, M. Poly (3-Hydroxybutyrate)-Multiwalled Carbon Nanotubes Electrospun Scaffolds Modified with Curcumin. Polymers 2020, 12, 2588. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Wu, Q.; Chen, J.; Chen, G.-Q. Evaluation of three-dimensional scaffolds made of blends of hydroxyapatite and poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) for bone reconstruction. Biomaterials 2005, 26, 899–904. [Google Scholar] [CrossRef]
- Hayati, A.N.; Rezaie, H.R.; Hosseinalipour, S.M. Preparation of poly (3-hydroxybutyrate)/nano-hydroxyapatite composite scaffolds for bone tissue engineering. Mater. Lett. 2011, 65, 736–739. [Google Scholar] [CrossRef]
- Kouhi, M.; Prabhakaran, M.P.; Shamanian, M.; Fathi, M.; Morshed, M.; Ramakrishna, S. Electrospun PHBV nanofibers containing HA and bredigite nanoparticles: Fabrication, characterization and evaluation of mechanical properties and bioactivity. Compos. Sci. Technol. 2015, 121, 115–122. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Jamshidi, A. A new strategy for fabrication of bone scaffolds using electrospun nano-HAp/PHB fibers and protein hydrogels. Chem. Eng. J. 2016, 289, 38–47. [Google Scholar] [CrossRef]
- Hayati, A.N.; Hosseinalipour, S.; Rezaie, H.; Shokrgozar, M.A. Characterization of poly (3-hydroxybutyrate)/nano-hydroxyapatite composite scaffolds fabricated without the use of organic solvents for bone tissue engineering applications. Mater. Sci. Eng. C 2012, 32, 416–422. [Google Scholar] [CrossRef]
- Chen, Z.; Song, Y.; Zhang, J.; Liu, W.; Cui, J.; Li, H. Laminated electrospun nHA/PHB-composite scaffolds mimicking bone extracellular matrix for bone tissue engineering. Mater. Sci. Eng. C 2017, 72, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Daranarong, D.; Chan, R.T.H.; Wanandy, N.S.; Molloy, R.; Punyodom, W.; Foster, L.J.R. Electrospun polyhydroxybutyrate and poly (L-lactide-co-ε-caprolactone) composites as nanofibrous scaffolds. BioMed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Manikandan, N.A.; Pakshirajan, K.; Pugazhenthi, G. Preparation and characterization of environmentally safe and highly biodegradable microbial polyhydroxybutyrate (PHB) based graphene nanocomposites for potential food packaging applications. Int. J. Biol. Macromol. 2020, 154, 866–877. [Google Scholar] [CrossRef]
- Pramanik, N.; Bhattacharya, S.; Rath, T.; De, J.; Adhikary, A.; Basu, R.K.; Kundu, P.P. Polyhydroxybutyrate-co-hydroxyvalerate copolymer modified graphite oxide based 3D scaffold for tissue engineering application. Mater. Sci. Eng. C 2019, 94, 534–546. [Google Scholar] [CrossRef]
- Bal, B.; Tugluca, I.B.; Koc, N.; Isoglu, I.A. On the detailed mechanical response investigation of PHBV/PCL and PHBV/PLGA electrospun mats. Mater. Res. Express 2019, 6, 065411. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M. Development and characterization of chitosan-grafted polycaprolactone/poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) fiber blends for tissue engineering applications. Int. J. Comput. Methods Exp. Meas. 2017, 5, 713–722. [Google Scholar] [CrossRef]
- Cai, Z.; Xiong, P.; He, S.; Zhu, C. Improved piezoelectric performances of highly orientated poly (β-hydroxybutyrate) electrospun nanofiber membrane scaffold blended with multiwalled carbon nanotubes. Mater. Lett. 2019, 240, 213–216. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, Y.; Wei, G.; Song, W.; Hu, T.; Yang, H.; Xue, R. Processing, structure, and properties of multiwalled carbon nanotube/poly (hydroxybutyrate-co-valerate) biopolymer nanocomposites. J. Appl. Polym. Sci. 2012, 125, E620–E629. [Google Scholar] [CrossRef]
- Liu, A.R.; Xu, B.Z.; Chen, C.C.; Huang, D.Y.; Liang, E.W.; Ge, F.X.; Ge, G.J. Effects of modified SWCNT on the mechanical and thermal properties of PLA/PHB bio-composites. AIP Adv. 2020, 10, 075122. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, M.; Zhang, J.; Zhang, Y.; Liu, Z.; Zhu, Y.; Zhang, C. Three dimensionally printed mesoporous bioactive glass and poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) composite scaffolds for bone regeneration. J. Mater. Chem. B 2014, 2, 6106–6118. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.; Xue, Z.; Li, H.; Liu, J. PHBV/bioglass composite scaffolds with co-cultures of endothelial cells and bone marrow stromal cells improve vascularization and osteogenesis for bone tissue engineering. RSC Adv. 2017, 7, 22197–22207. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Wang, J.; Tang, L.; Ao, H.; Tan, H.; Tang, T.; Liu, C. Mesoporous bioactive glass doped-poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) composite scaffolds with 3-dimensionally hierarchical pore networks for bone regeneration. Colloids Surf. B Biointerfaces 2014, 116, 72–80. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Prabhakaran, M.P.; Bahrami, S.H.; Ramakrishna, S. Gum tragacanth/poly (l-lactic acid) nanofibrous scaffolds for application in regeneration of peripheral nerve damage. Carbohydr. Polym. 2016, 140, 104–112. [Google Scholar] [CrossRef]
- Landel, R.F.; Nielsen, L.E. Mechanical Properties of Polymers and Composites; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Bleach, N.C.; Nazhat, S.N.; Tanner, K.E.; Kellomäki, M.; Törmälä, P. Effect of filler content on mechanical and dynamic mechanical properties of particulate biphasic calcium phosphate—Polylactide composites. Biomaterials 2002, 23, 1579–1585. [Google Scholar] [CrossRef]
- Iron, R.; Mehdikhani, M.; Naghashzargar, E.; Karbasi, S.; Semnani, D. Effects of nano-bioactive glass on structural, mechanical and bioactivity properties of Poly (3-hydroxybutyrate) electrospun scaffold for bone tissue engineering applications. Mater. Technol. 2019, 34, 540–548. [Google Scholar] [CrossRef]
- Sabir, M.I.; Xu, X.; Li, L. A review on biodegradable polymeric materials for bone tissue engineering applications. J. Mater. Sci. 2009, 44, 5713–5724. [Google Scholar] [CrossRef]
- Jack, K.; Velayudhan, S.; Luckman, P.; Trau, M.; Grøndahl, L.; Cooper-White, J. The fabrication and characterization of biodegradable HA/PHBV nanoparticle–polymer composite scaffolds. Acta Biomater. 2009, 5, 2657–2667. [Google Scholar] [CrossRef] [PubMed]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical applications of biodegradable polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freier, T.; Kunze, C.; Nischan, C.; Kramer, S.; Sternberg, K.; Sass, M.; Hopt, U.T.; Schmitz, K.-P. In vitro and in vivo degradation studies for development of a biodegradable patch based on poly (3-hydroxybutyrate). Biomaterials 2002, 23, 2649–2657. [Google Scholar] [CrossRef]
- Valappil, S.P.; Misra, S.K.; Boccaccini, A.R.; Roy, I. Biomedical applications of polyhydroxyalkanoates, an overview of animal testing and in vivo responses. Expert Rev. Med. Devices 2006, 3, 853–868. [Google Scholar] [CrossRef]
- Veleirinho, B.; Coelho, D.S.; Dias, P.F.; Maraschin, M.; Ribeiro-Do-Valle, R.M.; Lopes-Da-Silva, J.A. Nanofibrous poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/chitosan scaffolds for skin regeneration. Int. J. Biol. Macromol. 2012, 51, 343–350. [Google Scholar] [CrossRef]
- Hajiali, H.; Hosseinalipour, M.; Karbasi, S.; Shokrgozar, M.A. The influence of bioglass nanoparticles on the biodegradation and biocompatibility of poly (3-hydroxybutyrate) scaffolds. Int. J. Artif. Organs 2012, 35, 1015–1024. [Google Scholar] [CrossRef]
- Zhijiang, C.; Yi, X.; Haizheng, Y.; Jia, J.; Liu, Y. Poly (hydroxybutyrate)/cellulose acetate blend nanofiber scaffolds: Preparation, characterization and cytocompatibility. Mater. Sci. Eng. C 2016, 58, 757–767. [Google Scholar] [CrossRef]
- Arrieta, M.; López, J.; López, D.; Kenny, J.M.; Peponi, L. Development of flexible materials based on plasticized electrospun PLA–PHB blends: Structural, thermal, mechanical and disintegration properties. Eur. Polym. J. 2015, 73, 433–446. [Google Scholar] [CrossRef]
- Panda, P.K. Environmental friendly lead-free piezoelectric materials. J. Mater. Sci. 2009, 44, 5049–5062. [Google Scholar] [CrossRef] [Green Version]
- Fukada, E.; Yasuda, I. On the piezoelectric effect of bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Gjelsvik, A. Bone remodeling and piezoelectricity—I. J. Biomech. 1973, 6, 69–77. [Google Scholar] [CrossRef]
- Telega, J.J.; Wojnar, R. Piezoelectric effects in biological tissues. J. Theor. Appl. Mech. 2002, 40, 723–759. [Google Scholar]
- Vasquez-Sancho, F.; Abdollahi, A.; Damjanovic, D.; Catalan, G. Flexoelectricity in bones. Adv. Mater. 2018, 30, 1705316. [Google Scholar] [CrossRef]
- Tichý, J.; Erhart, J.; Kittinger, E.; Privratska, J. Fundamentals of Piezoelectric Sensorics: Mechanical, Dielectric, and Thermodynamical Properties of Piezoelectric Materials; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Sodano, H.A.; Inman, D.J.; Park, G. A review of power harvesting from vibration using piezoelectric materials. Shock. Vib. Dig. 2004, 36, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Rajabi, A.H.; Jaffe, M.; Arinzeh, T.L. Piezoelectric materials for tissue regeneration: A review. Acta Biomater. 2015, 24, 12–23. [Google Scholar] [CrossRef] [Green Version]
- Fukada, E.; Ando, Y. Piezoelectric properties of poly-β-hydroxybutyrate and copolymers of β-hydroxybutyrate and β-hydroxyvalerate. Int. J. Biol. Macromol. 1986, 8, 361–366. [Google Scholar] [CrossRef]
- Jacob, J.; More, N.; Mounika, C.; Gondaliya, P.; Kalia, K.; Kapusetti, G. Smart Piezoelectric Nanohybrid of Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and Barium Titanate for Stimulated Cartilage Regeneration. ACS Appl. Bio Mater. 2019, 2, 4922–4931. [Google Scholar] [CrossRef]
- Chernozem, R.V.; Surmeneva, M.A.; Surmenev, R.A. Hybrid biodegradable scaffolds of piezoelectric polyhydroxybutyrate and conductive polyaniline: Piezocharge constants and electric potential study. Mater. Lett. 2018, 220, 257–260. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [Green Version]
- Baldino, L.; Cardea, S.; Maffulli, N.; Reverchon, E. Regeneration techniques for bone-to-tendon and muscle-to-tendon interfaces reconstruction. Br. Med. Bull. 2016, 117, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Causa, F.; Netti, P.A.; Ambrosio, L.; Ciapetti, G.; Baldini, N.; Pagani, S. Poly-ϵ-caprolactone/hydroxyapatite composites for bone regeneration: In vitro characterization and human osteoblast response. J. Biomed. Mater. Res. Part A 2006, 76, 151–162. [Google Scholar] [CrossRef]
- Kim, H.-W.; Lee, H.-H.; Knowles, J.C. Electrospinning biomedical nanocomposite fibers of hydroxyapatite/poly (lactic acid) for bone regeneration. J. Biomed. Mater. Res. Part A 2006, 79, 643–649. [Google Scholar] [CrossRef]
- Jeong, S.I.; Ko, E.K.; Yum, J.; Jung, C.H.; Lee, Y.M.; Shin, H. Nanofibrous poly (lactic acid)/hydroxyapatite composite scaffolds for guided tissue regeneration. Macromol. Biosci. 2008, 8, 328–338. [Google Scholar] [CrossRef]
- Xu, J.; Khor, K.A.; Sui, J.; Chen, W. Preparation and characterization of a novel hydroxyapatite/carbon nanotubes composite and its interaction with osteoblast-like cells. Mater. Sci. Eng. C 2009, 29, 44–49. [Google Scholar] [CrossRef]
- Harle, J.; Kim, H.-W.; Mordan, N.; Knowles, J.C.; Salih, V. Initial responses of human osteoblasts to sol–gel modified titanium with hydroxyapatite and titania composition. Acta Biomater. 2006, 2, 547–556. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Jamshidi, A.; Irani, S. Nano-hydroxyapatite reinforced polyhydroxybutyrate composites: A comprehensive study on the structural and in vitro biological properties. Mater. Sci. Eng. C 2013, 33, 2776–2787. [Google Scholar] [CrossRef]
- Ramier, J.; Grande, D.; Bouderlique, T.; Stoilova, O.; Manolova, N.; Rashkov, I.; Renard, E. From design of bio-based biocomposite electrospun scaffolds to osteogenic differentiation of human mesenchymal stromal cells. J. Mater. Sci. Mater. Med. 2014, 25, 1563–1575. [Google Scholar] [CrossRef] [PubMed]
- Degli Esposti, M.; Chiellini, F.; Bondioli, F.; Morselli, D.; Fabbri, P. Highly porous PHB-based bioactive scaffolds for bone tissue engineering by in situ synthesis of hydroxyapatite. Mater. Sci. Eng. C 2019, 100, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Paşcu, E.I.; Stokes, J.; McGuinness, G.B. Electrospun composites of PHBV, silk fibroin and nano-hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. C 2013, 33, 4905–4916. [Google Scholar] [CrossRef] [PubMed]
- Rașoga, O.; Sima, L.; Chirițoiu, M.; Popescu-Pelin, G.; Fufă, O.; Grumezescu, V.; Socol, G. Biocomposite coatings based on Poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/calcium phosphates obtained by MAPLE for bone tissue engineering. Appl. Surf. Sci. 2017, 417, 204–212. [Google Scholar] [CrossRef]
- Zhang, S.; Prabhakaran, M.P.; Qin, X.; Ramakrishna, S. Biocomposite scaffolds for bone regeneration: Role of chitosan and hydroxyapatite within poly-3-hydroxybutyrate-co-3-hydroxyvalerate on mechanical properties and in vitro evaluation. J. Mech. Behav. Biomed. Mater. 2015, 51, 88–98. [Google Scholar] [CrossRef]
- Mauch, C.; Hatamochi, A.; Scharffetter, K.; Krieg, T. Regulation of collagen synthesis in fibroblasts within a three-dimensional collagen gel. Exp. Cell Res. 1988, 178, 493–503. [Google Scholar] [CrossRef]
- Park, J.-C.; Hwang, Y.-S.; Lee, J.-E.; Park, K.D.; Matsumura, K.; Hyon, S.-H.; Suh, H. Type I atelocollagen grafting onto ozone-treated polyurethane films: Cell attachment, proliferation, and collagen synthesis. J. Biomed. Mater. Res. 2000, 52, 669–677. [Google Scholar] [CrossRef]
- Nehrer, S.; Breinan, H.A.; Ramappa, A.; Hsu, H.P.; Minas, T.; Shortkroff, S.; Spector, M. Chondrocyte-seeded collagen matrices implanted in a chondral defect in a canine model. Biomaterials 1998, 19, 2313–2328. [Google Scholar] [CrossRef]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolym. Orig. Res. Biomol. 2008, 89, 338–344. [Google Scholar] [CrossRef]
- Baek, J.-Y.; Xing, Z.-C.; Kwak, G.; Yoon, K.-B.; Park, S.-Y.; Park, L.S.; Kang, I.-K. Fabrication and characterization of collagen-immobilized porous PHBV/HA nanocomposite scaffolds for bone tissue engineering. J. Nanomater. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Schrooten, J.; Helsen, J. Adhesion of bioactive glass coating to Ti6Al4V oral implant. Biomaterials 2000, 21, 1461–1469. [Google Scholar] [CrossRef]
- Montazeri, M.; Karbasi, S.; Foroughi, M.R.; Monshi, A.; Ebrahimi-Kahrizsangi, R. Evaluation of mechanical property and bioactivity of nano-bioglass 45S5 scaffold coated with poly-3-hydroxybutyrate. J. Mater. Sci. Mater. Med. 2015, 26, 62. [Google Scholar] [CrossRef]
- Mikael, P.E.; Amini, A.R.; Basu, J.; Arellano-Jimenez, M.J.; Laurencin, C.T.; Sanders, M.M.; Nukavarapu, S.P. Functionalized carbon nanotube reinforced scaffolds for bone regenerative engineering: Fabrication, in vitro and in vivo evaluation. Biomed. Mater. 2014, 9, 035001. [Google Scholar] [CrossRef]
- Antonioli, E.; Lobo, A.O.; Ferretti, M.; Cohen, M.; Marciano, F.R.; Corat, E.J.; Trava-Airoldi, V.J. An evaluation of chondrocyte morphology and gene expression on superhydrophilic vertically-aligned multi-walled carbon nanotube films. Mater. Sci. Eng. C 2013, 33, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Mankin, H.J. Articular cartilage: Tissue design and chondrocyte-matrix interactions. Instr. Course Lect. 1998, 47, 477. [Google Scholar] [PubMed]
- Karkhaneh, A.; Naghizadeh, Z.; Shokrgozar, M.A.; Bonakdar, S.; Solouk, A.; Haghighipour, N. Effects of hydrostatic pressure on biosynthetic activity during chondrogenic differentiation of MSCs in hybrid scaffolds. Int. J. Artif. Organs 2014, 37, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, B.; Alini, M.; Cucchiarini, M.; Dodge, G.R.; Eglin, D.; Guilak, F.; Semino, C.E. Tissue engineering for articular cartilage repair—The state of the art. Eur. Cells Mater. 2013, 25, e67. [Google Scholar] [CrossRef]
- Lahiji, A.; Sohrabi, A.; Hungerford, D.S.; Frondoza, C.G. Chitosan supports the expression of extracellular matrix proteins in human osteoblasts and chondrocytes. J. Biomed. Mater. Res. 2000, 51, 586–595. [Google Scholar] [CrossRef]
- Suh, J.-K.F.; Matthew, H.W.T. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar]
- Giretova, M.; Medvecky, L.; Petrovova, E.; Cizkova, D.; Danko, J.; Mudronova, D.; Slovinska, L.; Bures, R. Polyhydroxybutyrate/chitosan 3D scaffolds promote in vitro and in vivo chondrogenesis. Appl. Biochem. Biotechnol. 2019, 189, 556–575. [Google Scholar] [CrossRef]
- Wu, J.; Xue, K.; Li, H.; Sun, J.; Liu, K. Improvement of PHBV scaffolds with bioglass for cartilage tissue engineering. PLoS ONE 2013, 8, e71563. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Y.-C.; Wang, C.-T. Neuronal differentiation of induced pluripotent stem cells in hybrid polyester scaffolds with heparinized surface. Colloids Surf. B Biointerfaces 2012, 100, 9–15. [Google Scholar] [CrossRef]
- Sahana, T.G.; Rekha, P.D. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef]
- Norouzi, M.; Boroujeni, S.M.; Omidvarkordshouli, N.; Soleimani, M. Advances in skin regeneration: Application of electrospun scaffolds. Adv. Health Mater. 2015, 4, 1114–1133. [Google Scholar] [CrossRef]
- MacEwan, M.R.; MacEwan, S.; Kovacs, T.R.; Batts, J. What makes the optimal wound healing material? A review of current science and introduction of a synthetic nanofabricated wound care scaffold. Cureus 2017, 9, e1736. [Google Scholar] [CrossRef] [Green Version]
- Pitzer, G.B.; Patel, K.G. Proper care of early wounds to optimize healing and prevent complications. Facial Plast. Surg. Clin. N. Am. 2011, 19, 491–504. [Google Scholar] [CrossRef]
- Medvecky, L.; Giretova, M.; Stulajterova, R. Properties and in vitro characterization of polyhydroxybutyrate–chitosan scaffolds prepared by modified precipitation method. J. Mater. Sci. Mater. Electron. 2014, 25, 777–789. [Google Scholar] [CrossRef]
- Ma, G.; Yang, D.; Wang, K.; Han, J.; Ding, S.; Song, G.; Nie, J. Organic-soluble chitosan/polyhydroxybutyrate ultrafine fibers as skin regeneration prepared by electrospinning. J. Appl. Polym. Sci. 2010, 118, 3619–3624. [Google Scholar] [CrossRef]
- Zhijiang, C.; Guang, Y.; Kim, J. Biocompatible nanocomposites prepared by impregnating bacterial cellulose nanofibrils into poly (3-hydroxybutyrate). Curr. Appl. Phys. 2011, 11, 247–249. [Google Scholar] [CrossRef]
- Nagiah, N.; Madhavi, L.; Anitha, R.; Anandan, C.; Srinivasan, N.T.; Sivagnanam, U.T. Development and characterization of coaxially electrospun gelatin coated poly (3-hydroxybutyric acid) thin films as potential scaffolds for skin regeneration. Mater. Sci. Eng. C 2013, 33, 4444–4452. [Google Scholar] [CrossRef]
- Vigneswari, S.; Murugaiyah, V.; Kaur, G.; Khalil, H.P.S.; Abdul Amirul, A.A. Simultaneous dual syringe electrospinning system using benign solvent to fabricate nanofibrous P (3HB-co-4HB)/collagen peptides construct as potential leave-on wound dressing. Mater. Sci. Eng. C 2016, 66, 147–155. [Google Scholar] [CrossRef]
- Daisy, E.A.C.; Rajendran, N.K.; Houreld, N.N.; Marraiki, N.; Elgorban, A.M.; Rajan, M. Curcumin and Gymnema sylvestre extract loaded graphene oxide-polyhydroxybutyrate-sodium alginate composite for diabetic wound regeneration. React. Funct. Polym. 2020, 154, 104671. [Google Scholar] [CrossRef]
- Mohanna, P.-N.; Terenghi, G.; Wiberg, M. Composite PHB-GGF conduit for long nerve gap repair: A long-term evaluation. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2005, 39, 129–137. [Google Scholar] [CrossRef]
- Young, R.C.; Terenghi, G.; Wiberg, M. Poly-3-hydroxybutyrate (PHB): A resorbable conduit for long-gap repair in peripheral nerves. Br. J. Plast. Surg. 2002, 55, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Mohanna, P.N.; Young, R.C.; Wiberg, M.; Terenghi, G. A composite poly-hydroxybutyrate–glial growth factor conduit for long nerve gap repairs. J. Anat. 2003, 203, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-T.; Sun, J.; Chen, S.; Chen, G.-Q. In vitro investigation of maleated poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) for its biocompatibility to mouse fibroblast L929 and human microvascular endothelial cells. J. Biomed. Mater. Res. Part A 2008, 87, 832–842. [Google Scholar] [CrossRef]
- Chernozem, R.V.; Guselnikova, O.; Surmeneva, M.A.; Postnikov, P.S.; Abalymov, A.A.; Parakhonskiy, B.V.; Surmenev, R.A. Diazonium chemistry surface treatment of piezoelectric polyhydroxybutyrate scaffolds for enhanced osteoblastic cell growth. Appl. Mater. Today 2020, 20, 100758. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V.A. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. eXPRESS Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef] [Green Version]
- Bonartsev, A.P.; Bonartseva, G.A.; Reshetov, I.V.; Kirpichnikov, M.P.; Shaitan, K.V. Application of polyhydroxyalkanoates in medicine and the biological activity of natural poly (3-hydroxybutyrate). Acta Nat. 2019, 11, 4–16. [Google Scholar] [CrossRef] [PubMed]














| Composite | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) | Ref. |
|---|---|---|---|---|
| PHBV | 5.82 ± 0.50 | 67.7 ± 5.2 | 50.2 ± 4.5 | [51] |
| 50PHBV/50SF | 3.87 ± 0.37 | 60.5 ± 5.0 | 29.8 ± 2.7 | |
| PHBHHx | 11.7 ± 0.5 | 204 ± 5 | [12] | |
| PHBHHx/SF | 11.5 ± 0.5 | 175 ± 5 | ||
| PHB | 6.23 ± 0.3 | 11.74 | [50] | |
| PHB/SF | 3.81 ± 0.1 | 17.10 | ||
| PHB | 87 ± 3.02 | 74.45 ± 2.88 | 26 ± 1.67 | [32] |
| PHB/10 wt% CTS | 63.66 ± 6.10 | 52.79 ± 4.52 | 46 ± 4.02 | |
| PHB/20 wt% CTS | 31.6 ± 3.37 | 50.74 ± 2.23 | 65.5 ± 2.25 | |
| Aligned PHB | 16.2 ± 3.11 | 202.1 ± 97.6 | 7.3 ± 0.8 | [52] |
| Aligned PHB/15 wt% CTS | 8.73 ± 3.65 | 210.2 ± 90.9 | 1.45 ± 0.67 | |
| Random PHB | 7.6 ± 0.8 | 164.3 ± 82.4 | 3.83 ± 0.69 | |
| Random PHB/15 wt% CTS | 6.41 ± 3.32 | 150.8 ± 93.6 | 1.19 ± 0.71 | |
| PHBV | 4.01 ± 0.27 | 108 ± 2.61 | 56.34 ± 2.66 | [14] |
| PHBV/Col 50:50 | 2.17 ± 0.27 | 70.55 ± 1.78 | 8.17 ± 1.60 | |
| PHBV | 94 | [11] | ||
| PHBV/GO | 254 | |||
| PHBV/GO/Collagen | 241 | |||
| PHB | 8.4 ± 1.9 | 554 ± 25 | 3.8 ± 1.2 | [80] |
| PHB/CTS | 8.7 ± 1.2 | 467 ± 22 | 84.1 ± 4.7 | |
| PHB/CTS/BCP | 16.5 ± 0.9 | 524 ± 20 | 99.2 ± 5.1 | |
| PHB | 3.8 | 11.71 | [54] | |
| PHB/CTS | 3.4 | |||
| PHB/CTS/1 wt% MWCNT | 10 | 20.99 | ||
| PHB | 10.67 ± 1.01 | 238 ± 52 | 7.27 ± 0.49 | [58] |
| PHB/nHA (blend) | 16.16 ± 0.86 | 397 ± 107 | 12.48 ± 1.57 | |
| PHB/nHA (spray) | 5.47 ± 0.18 | 138 ± 19 | 4.90 ± 0.25 | |
| PHBV | 4.41 ± 0.27 | 106.70 ± 31.33 | [84] | |
| PHBV/10 nHABR | 6.35 ± 0.38 | 158.60 ± 34.67 | ||
| PHB | 1.2 ± 0.2 | 10.6 ± 1.4 | [88] | |
| PHB/25 wt% PLCL | 1.2 ± 0.2 | 41.6 ± 0.8 | ||
| PHBV (100 wt%, w/w) | 0.1 | 0.34 | 108.32 | [91] |
| PHBV/PLGA (50:50 wt%, w/w) | 4.65 | 47 | 125.65 | |
| PHBV/PCL (50:50 wt%, w/w) | 2.56 | 20.63 | 115 | |
| PHBV/PCL (50:50 wt%, w/w)+ 1 wt% CA | 1.55 | 7.47 | 210 | |
| PHBV/PCL (50:50 wt%, w/w) + 10 wt% CA | 1.2 | 7.44 | 43 | |
| PHB | 18.8 | 7 | [64] | |
| 40PHB/60PCL | 26.9 | 1358 | ||
| PHBHHx | 10 | 220 | 102 | [92] |
| 50PHBHHx/50CS-g-PCL | 19 | 390 | 148 | |
| PHB | 2 | 108 | [16] | |
| PHB/0.5 wt% CNT | 5.15 | 285 | ||
| 60PLA/40PHB | 11.8 | 43.8 | [95] | |
| 60PLA/40PHB/0.1wt% HACNT | 27.87 | 346.68 | ||
| PHB | 12.4 | [93] | ||
| PHB/MWCNT | 16.2 | |||
| PHB/MWCNT/hot stretching | 21.7 | |||
| PHB | 1.13 ± 0.021 | 99.41 ± 2.88 | [102] | |
| PHB/7.5 wt% nBG | 1.91 ± 1.00 | 30.59 | ||
| Cancellous bone | 2–12 | 20–500 | [28,103] | |
| Cortical bone | 100–230 | 3000–30,000 | ||
| Cartilages | 3.7–10.5 | 0.7–15.3 | [103] | |
| Composite | Compressive Strength (MPa) | Compressive Young’s Modulus (MPa) | Ref |
|---|---|---|---|
| PHB | 22 ± 2 | 317 ± 70 | [82] |
| PHB/mHA | 30 ± 6 | 419 ± 80 | |
| PHBHHx | 8 ± 1 | 173 ± 49 | |
| PHBHHx/mHA | 8 ± 1 | 68 ± 5 | |
| PHB | 2.14 ± 0.11 | 22.16 ± 2.75 | [83] |
| PHB/10wt% nHA | 3.18 ± 0.24 | 41.33 ± 3.21 | |
| PHB | 2.03 ± 0.14 | 29.06 ± 2.74 | [86] |
| PHB/5 wt% nHA | 2.38 ± 0.13 | 36.91 ± 3.12 | |
| PHB/10 wt% nHA | 2.76 ± 0.18 | 45.73 ± 3.87 | |
| PHB/15 wt% nHA | 3.19 ± 0.21 | 56.12 ± 4.28 | |
| PHBV | 0.15 ± 0.02 | [97] | |
| PHBV/10 wt% mBG | 0.25 ± 0.04 | ||
| PHBV/20 wt% mBG | 0.32 ± 0.03 | ||
| Cancellous bone | 2–12 | 50–500 | [104] |
| Cortical bone | 100–200 | 7000–30,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pryadko, A.; Surmeneva, M.A.; Surmenev, R.A. Review of Hybrid Materials Based on Polyhydroxyalkanoates for Tissue Engineering Applications. Polymers 2021, 13, 1738. https://doi.org/10.3390/polym13111738
Pryadko A, Surmeneva MA, Surmenev RA. Review of Hybrid Materials Based on Polyhydroxyalkanoates for Tissue Engineering Applications. Polymers. 2021; 13(11):1738. https://doi.org/10.3390/polym13111738
Chicago/Turabian StylePryadko, Artyom, Maria A. Surmeneva, and Roman A. Surmenev. 2021. "Review of Hybrid Materials Based on Polyhydroxyalkanoates for Tissue Engineering Applications" Polymers 13, no. 11: 1738. https://doi.org/10.3390/polym13111738
APA StylePryadko, A., Surmeneva, M. A., & Surmenev, R. A. (2021). Review of Hybrid Materials Based on Polyhydroxyalkanoates for Tissue Engineering Applications. Polymers, 13(11), 1738. https://doi.org/10.3390/polym13111738