Estimation of the Effect of Accelerating New Bone Formation of High and Low Molecular Weight Hyaluronic Acid Hybrid: An Animal Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
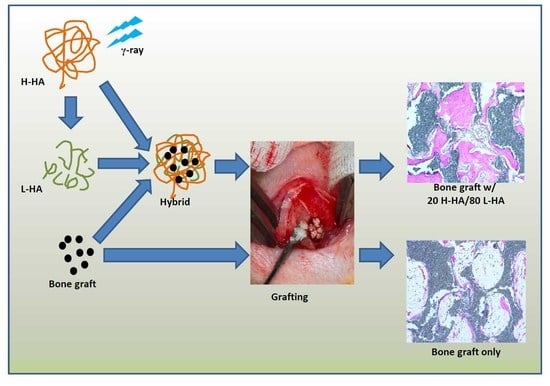
2.2. Preparation of H-HA/L-HA Hybrids
2.3. Physicochemical Properties Tests of the H-HA/L-HA Hybrid
2.4. Animal Experiment
2.5. Micro-CT Measurements
2.6. Histological and Histomorphometrical Evaluation
2.7. Statistical Analysis
3. Results and Discussions
Characterization Results for Fe3O4 NPs
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Schwartz, Z.; Goldstein, M.; Raviv, E.; Hirsch, A.; Ranly, D.M.; Boyan, B.D. Clinical evaluation of demineralized bone allograft in a hyaluronic acid carrier for sinus lift augmentation in humans: A computed tomography and histo-morphometric study. Clin. Oral Implant. Res. 2007, 18, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.C.; Korn, P.; Stadlinger, B.; Range, U.; Möller, S.; Becher, J.; Schnabelrauch, M.; Mai, R.; Scharnweber, D.; Eckelt, U.; et al. Coatingwith artificial matrices from collagen and sulfated hyaluronan influences the osseointegration of dental implants. J. Mater. Sci. Mater. Med. 2014, 25, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Korn, P.; Schulz, M.C.; Hintze, V.; Range, U.; Mai, R.; Eckelt, U.; Schnabelrauch, M.; Möller, S.; Becher, J.; Scharnweber, D.; et al. Chondroitin sulfate and sulfated hyaluronan-containing collagen coatings of titanium implants influence peri-implant bone formation in a minipig model. J. Biomed. Mater. Res. A 2014, 102, 2334–2344. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.R.; Moreira-Teixeira, L.S.; Moroni, L.; Reis, R.L.; van Blitterswijk, C.A.; Karperien, M.; Mano, J.F. Chitosan scaffolds containing hyaluronic acid for cartilage tissue engineering. Tissue Eng. Part C Methods 2011, 17, 717–730. [Google Scholar] [CrossRef] [Green Version]
- Mathews, S.; Bhonde, R.; Gupta, P.K.; Totey, S. Novel biomimetic tripolymer scaffolds consisting of chitosan, collagen type 1, and hyaluronic acid for bone marrow-derived human mesenchymal stem cells-based bone tissue engineering. J. Biomed. Mater. Res. B 2014, 102, 1825–1834. [Google Scholar] [CrossRef]
- Nguyen, T.B.L.; Lee, B.T. A combination of biphasic calcium phosphate scaffold with hyaluronic acid-gelatin hydrogel as a new tool for bone regeneration. Tissue Eng. Part A 2014, 20, 1993–2004. [Google Scholar] [CrossRef] [Green Version]
- Dogan, E.; Dursun, E.; Tosun, E.; Bilgic, E.; Akman, A.C.; Orhan, K.; Celik, H.H.; Korkusuz, P.; Caglayan, F. Evaluation of hyaluronic matrix efficacy in sinus augmentation: A randomized-controlled histomorphometric and micro-computed tomography analysis. Int. J. Oral Maxillofac. Surg. 2017, 46, 931–937. [Google Scholar] [CrossRef]
- Chang, Y.L.; LO, Y.J.; Feng, S.W.; Huang, Y.C.; Tsai, H.Y.; Lin, C.T.; Fan, K.H.; Huang, H.M. Bone healing improvements using hyaluronic acid and hydroxyapatite/beta-tricalcium phosphate in combination: An animal study. BioMed Res. Int. 2016, 2016, 8301624. [Google Scholar] [CrossRef]
- Elkarargy, A. Alveolar sockets preservation using hydroxyapatite / beta tricalcium phosphate with hyaluronic acid (Histomorphometric study). J. Am. Sci. 2013, 9, 556–563. [Google Scholar]
- Aguado, E.; Pascaretti-Grizon, F.; Gaudin-Audrain, C.; Goyenvalle, E.; Chappard, D. β-TCP granules mixed with reticulated hyaluronic acid induce an increase in bone apposition. Biomed. Mater. 2014, 9, 015001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelo, T.; Marcel, W.; Andreas, K.; Izabela, S. Biomechanical stability of dental implants in augmented maxillary sites: Results of a randomized clinical study with four different biomaterials and PRF and a biological view on guided bone regeneration. BioMed Res. Int. 2015, 2015, 850340. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Watanabe, C. Stimulation of osteoinduction in bone wound healing by high-molecular hyaluronic acid. Bone 1995, 16, 9–15. [Google Scholar] [CrossRef]
- Hempel, U.; Möller, S.; Noack, C.; Hintze, V.; Scharnweber, D.; Schnabelrauch, M.; Dieter, P. Sulfated hyaluronan/collagen I matrices enhance the osteogenic differentiation of human mesenchymal stromal cells in vitro even in the absence of dexamethasone. Acta Biomater. 2012, 8, 4064–4072. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Zhou, C.; Hui, D.; Du, C.; Wu, L.; Wang, L.; Wang, W.; Pu, X.; Gu, L.; Liu, L.; et al. Hyaluronic acid as a bioactive component for bone tissue regeneration: Fabrication, modification, properties, and biological functions. Nanotechnol. Rev. 2020, 9, 1059–1079. [Google Scholar] [CrossRef]
- Zou, L.; Zou, X.; Chen, L.; Li, H.; Mygind, T.; Kassem, M.; Bünger, C. Effect of hyaluronan on osteogenic differentiation of porcine bone marrow stromal cells in vitro. J. Orthop. Res. 2008, 26, 713–720. [Google Scholar] [CrossRef]
- Zhao, N.; Wang, X.; Qin, L.; Guo, Z.; Li, D. Effect of molecular weight and concentration of hyaluronan on cell proliferation and osteogenic differentiation in vitro. Biochem. Biophys. Res. Commun. 2015, 465, 569–574. [Google Scholar] [CrossRef]
- D’Agostino, A.; Stellavato, A.; Busico, T.; Papa, T.; Tirino, V.; Papaccio, G.; La Gatta, A.; De Rosa, M.; Schiraldi, C. In Vitro analysis of the effects on wound healing of high and low-molecular weight chains of hyaluronan and their hybrid H-HA/L-HA complexes. BMC Mol. Cell Biol. 2015, 16, 19. [Google Scholar] [CrossRef] [Green Version]
- Trabucchi, E.; Pallotta, S.; Morini, M.; Corsi, F.; Franceschini, R.; Casiraghi, A.; Pravettoni, A.; Foschi, D.; Minghetti, P. Low molecular weight hyaluronic acid prevents oxygen free radical damage to granulation tissue during wound healing. Int. J. Tissue React. 2002, 24, 65–71. [Google Scholar]
- Huang, Y.C.; Huang, K.Y.; Lew, W.Z.; Fan, K.H.; Chang, W.J.; Huang, H.M. Gamma-irradiation-prepared low molecular weight hyaluronic acid promotes skin wound healing. Polymers 2019, 11, 1214. [Google Scholar] [CrossRef] [Green Version]
- Ariyoshi, W.; Takahashi, T.; Kanno, T.; Ichimiya, H.; Takano, H.; Koseki, T.; Nishihara, T. Mechanisms involved in enhancement of osteoclast formation and function by low molecular weight hyaluronic acid. J. Biol. Chem. 2005, 280, 18967–18972. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Park, Y.; Tae, G.; Lee, K.B.; Hwang, C.M.; Hwang, S.J.; Kim, I.S.; Noh, I.; Sun, K. Characterization of low-molecular-weight hyaluronic acid-based hydrogel and differential stem cell responses in the hydrogel microenvironments. J. Biomed. Mater. Res. A 2009, 88, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Fagien, S.; Bertucci, V.; von Grote, E.; Mashburn, J.H. Rheologic and physicochemical properties used to differentiate injectable hyaluronic acid filler products. Plast. Reconstr. Surg. 2019, 143, 707–720. [Google Scholar] [CrossRef]
- Tamimi, F.M.; Torres, J.; Tresguerres, I.; Clemente, C.; L´opez-Cabarcos, E.; Blanco, L.J. Bone augmentation in rabbit calvariae: Comparative study between Bio-Oss® and a novel β-TCP/DCPD granulate. J. Clin. Periodontol. 2006, 33, 922–928. [Google Scholar] [CrossRef] [Green Version]
- Andersson, L.; Ramzi, A.; Joseph, B. Studies on dentin grafts to bone defects in rabbit tibia and mandible; development of an experimental model. Dent. Traumatol. 2009, 25, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qin, J.; Hu, Y. Efficient degradation of high-molecular-weight hyaluronic acid by a combination of ultrasound, hydrogen peroxide, and copper ion. Molecules 2019, 24, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.; Kim, J.K.; Kim, J.H.; Kweon, D.K.; Lee, J.W. Degradation of hyaluronic acid powder by electron beam irradiation, gamma ray irradiation, microwave irradiation and thermal treatment: A comparative study. Carbohydr. Polym. 2010, 79, 1080–1085. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic acid: The influence of molecular weight on structural, physical, physico-chemical, and degradable properties of biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y. Preparation of low-molecular-weight hyaluronic acid by ozone treatment. Carbohydr. Polym. 2012, 89, 709–712. [Google Scholar] [CrossRef]
- Gura, E.; Hückel, M.; Müller, P.J. Specific degradation of hyaluronic acid and its rheological properties. Polym. Degrad. Stab. 1998, 59, 297–302. [Google Scholar] [CrossRef]
- Stellavato, A.; Vassallo, V.; La Gatta, A.; Pirozzi, A.V.A.; De Rosa, M.; Balato, G.; D’Addona, A.; Tirino, V.; Ruosi, C.; Schiraldi, C. Novel hybrid gels made of high and low molecular weight hyaluronic acid induce proliferation and reduce inflammation in an osteoarthritis in vitro model based on human synoviocytes and chondrocytes. BioMed Res. Int. 2019, 2019, 4328219. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Chen, H.; Xu, C.; Yu, D.; Xu, H.; Hu, Y. Synthesis of hyaluronic acid hydrogels by crosslinking the mixture of high-molecular-weight hyaluronic acid and low-molecular-weight hyaluronic acid with 1,4-butanediol diglycidyl ether. RSC Adv. 2020, 10, 7206–7213. [Google Scholar] [CrossRef]
- Wang, H.T.; Chou, P.C.; Wu, P.H.; Lee, C.M.; Fan, K.H.; Chang, W.J.; Lee, S.Y.; Huang, H.M. Physical and biological evaluation of low-molecular-weight hyaluronic acid-modified Fe3O4 nanoparticle for targeting MCF7 breast cancer cells. Polymers 2020, 12, 1094. [Google Scholar] [CrossRef]
- Lai, J.Y.; Tu, I.H. Adhesion, phenotypic expression, and biosynthetic capacity of corneal keratocytes on surfaces coated with hyaluronic acid of different molecular weights. Acta Biomater. 2012, 8, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Lapčík, L., Jr.; Benešová, K.; Lapčík, L.; De Smedt, S.; Lapčíková, B. Chemical modification of hyaluronic acid: Alkylation. Int. J. Polym. Anal. Charact. 2010, 15, 486–496. [Google Scholar] [CrossRef]
- Ellinger, R.F.; Nery, E.B.; Lynch, K.L. Histological assessment of periodontal osseous defects following implantation of hydroxyapatite and biphasic calciumphosphate ceramics: A case report. Int. J. Periodontics Restor. Dent. 1986, 6, 22–33. [Google Scholar]
- Daculsi, G.; LeGeros, R.Z.; Nery, E.; Lynch, K.; Kerebel, B. Transformation of biphasic calcium phosphate ceramics in vivo: Ultrastructural and physicochemical characterization. J. Biomed. Mater. Res. 1989, 23, 883–894. [Google Scholar] [CrossRef]
- Nery, E.B.; LeGeros, R.Z.; Lynch, K.L.; Lee, K. Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/beta TCP in periodontal osseous defects. J. Periodontol. 1992, 63, 729–735. [Google Scholar] [CrossRef]
- Park, K. Injectable hyaluronic acid hydrogel for bone augmentation. J. Control. Release 2011, 152, 207–208. [Google Scholar] [CrossRef]
- Tan, H.; Li, H.; Rubin, J.P.; Marra, K.G. Controlled gelation and degradation rates of injectable hyaluronic acid-based hydrogels through a double crosslinking strategy. J. Tissue Eng. Regen. Med. 2011, 5, 790–797. [Google Scholar] [CrossRef] [Green Version]
- Stellavato, A.; Corsuto, L.; D’Agostino, A.; La Gatta, A.; Diana, P.; Bernini, P.; De Rosa, M.; Schiraldi, C. Hyaluronan hybrid cooperative complexes as a novel frontier for cellular bioprocesses re-activation. PLoS ONE 2016, 11, e0163510. [Google Scholar] [CrossRef] [Green Version]
- Stern, R.; Asari, A.A.; Sugahara, K.N. Hyaluronan fragments: An information-rich system. Eur. J. Cell Biol. 2006, 85, 699–715. [Google Scholar] [CrossRef] [PubMed]
- La Gatta, A.; Corsuto, L.; Salzillo, R.; D’Agostino, A.; De Rosa, M.; Bracco, A.; Schiraldi, C. In vitro evaluation of hybrid cooperative complexes of hyaluronic acid as a potential new ophthalmic treatment. J. Ocul. Pharmacol. Ther. 2018, 34, 677–684. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, P.-J.; Yen, H.-J.; Lin, C.-Y.; Lai, H.-Y.; Chen, C.-H.; Wang, S.-H.; Chang, W.-J.; Lee, S.-Y.; Huang, H.-M. Estimation of the Effect of Accelerating New Bone Formation of High and Low Molecular Weight Hyaluronic Acid Hybrid: An Animal Study. Polymers 2021, 13, 1708. https://doi.org/10.3390/polym13111708
Kuo P-J, Yen H-J, Lin C-Y, Lai H-Y, Chen C-H, Wang S-H, Chang W-J, Lee S-Y, Huang H-M. Estimation of the Effect of Accelerating New Bone Formation of High and Low Molecular Weight Hyaluronic Acid Hybrid: An Animal Study. Polymers. 2021; 13(11):1708. https://doi.org/10.3390/polym13111708
Chicago/Turabian StyleKuo, Po-Jan, Hsiu-Ju Yen, Chi-Yu Lin, Hsuan-Yu Lai, Chun-Hung Chen, Shwu-Huey Wang, Wei-Jen Chang, Sheng-Yang Lee, and Haw-Ming Huang. 2021. "Estimation of the Effect of Accelerating New Bone Formation of High and Low Molecular Weight Hyaluronic Acid Hybrid: An Animal Study" Polymers 13, no. 11: 1708. https://doi.org/10.3390/polym13111708
APA StyleKuo, P. -J., Yen, H. -J., Lin, C. -Y., Lai, H. -Y., Chen, C. -H., Wang, S. -H., Chang, W. -J., Lee, S. -Y., & Huang, H. -M. (2021). Estimation of the Effect of Accelerating New Bone Formation of High and Low Molecular Weight Hyaluronic Acid Hybrid: An Animal Study. Polymers, 13(11), 1708. https://doi.org/10.3390/polym13111708