Interaction of Poly L-Lactide and Tungsten Disulfide Nanotubes Studied by In Situ X-ray Scattering during Expansion of PLLA/WS2NT Nanocomposite Tubes
Abstract
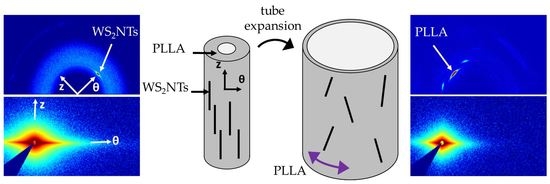
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Tungsten Disulfide Nanotubes (WS2NTs)
2.2. Tube Extrusion
2.3. Tube Expansion Instrument
2.4. In Situ Structural Characterization
2.5. Expansion Parameters
2.6. Measurement of the Tube Thickness
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanemann, T.; Szabó, D.V. Polymer-Nanoparticle Composites: From Synthesis to Modern Applications. Materials 2010, 3, 3468–3517. [Google Scholar] [CrossRef]
- Li, S.; Lin, M.M.; Toprak, M.S.; Kim, D.K.; Muhammed, M. Nanocomposites of polymer and inorganic nanoparticles for optical and magnetic applications. Nano Rev. 2010, 1, 5214. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Winey, K.I. Polymer Nanocomposites Containing Carbon Nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- Kumar, S.K.; Benicewicz, B.C.; Vaia, R.A.; Winey, K.I. 50th Anniversary Perspective: Are Polymer Nanocomposites Practical for Applications? Macromolecules 2017, 50, 714–731. [Google Scholar] [CrossRef]
- Wagner, H.D.; Vaia, R.A. Nanocomposites: Issues at the interface. Mater. Today 2004, 7, 38–42. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Sun, Z.; Huang, P.; Li, Y.-Q.; Hu, N. Some basic aspects of polymer nanocomposites: A critical review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Feng, X.-Q.; Lauke, B.; Mai, Y.-W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Atif, R.; Inam, F. Reasons and remedies for the agglomeration of multilayered graphene and carbon nanotubes in polymers. Beilstein J. Nanotechnol. 2016, 7, 1174–1196. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.; Saphiannikova, M.; Jehnichen, D.; Bierdel, M.; Heinrich, G. Experimental and theoretical studies of agglomeration effects in multi-walled carbon nanotube-polycarbonate melts. Express Polym. Lett. 2009, 3, 753–768. [Google Scholar] [CrossRef]
- Mout, R.; Moyano, D.F.; Rana, S.; Rotello, V.M. Surface functionalization of nanoparticles for nanomedicine. Chem. Soc. Rev. 2012, 41, 2539–2544. [Google Scholar] [CrossRef]
- Villmow, T.; Kretzschmar, B.; Pötschke, P. Influence of screw configuration, residence time, and specific mechanical energy in twin-screw extrusion of polycaprolactone/multi-walled carbon nanotube composites. Compos. Sci. Technol. 2010, 70, 2045–2055. [Google Scholar] [CrossRef] [Green Version]
- Abu-Zurayk, R.; Harkin-Jones, E.; McNally, T.; Menary, G.; Martin, P.; Armstrong, C. Biaxial deformation behavior and mechanical properties of a polypropylene/clay nanocomposite. Compos. Sci. Technol. 2009, 69, 1644–1652. [Google Scholar] [CrossRef]
- Mayoral, B.; Hornsby, P.R.; McNally, T.; Schiller, T.L.; Jack, K.; Martin, D.J. Quasi-solid state uniaxial and biaxial deformation of PET/MWCNT composites: Structural evolution, electrical and mechanical properties. RSC Adv. 2013, 3, 5162–5183. [Google Scholar] [CrossRef]
- Ning, N.; Fu, S.; Zhang, W.; Chen, F.; Wang, K.; Deng, H.; Zhang, Q.; Fu, Q. Realizing the enhancement of interfacial interaction in semicrystalline polymer/filler composites via interfacial crystallization. Prog. Polym. Sci. 2012, 37, 1425–1455. [Google Scholar] [CrossRef]
- Lim, L.-T.; Auras, R.; Rubino, M. Processing technologies for poly (lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [Green Version]
- Davachi, S.M.; Kaffashi, B. Polylactic Acid in Medicine. Polym. Technol. Eng. 2015, 54, 944–967. [Google Scholar] [CrossRef]
- Ailianou, A.; Ramachandran, K.; Kossuth, M.B.; Oberhauser, J.P.; Kornfield, J.A. Multiplicity of morphologies in poly (l-lactide) bioresorbable vascular scaffolds. Proc. Natl. Acad. Sci. USA 2016, 113, 11670–11675. [Google Scholar] [CrossRef] [Green Version]
- Kossuth, M.B.; Perkins, L.E.; Rapoza, R.J. Design Principles of Bioresorbable Polymeric Scaffolds. Interv. Cardiol. Clin. 2016, 5, 349–355. [Google Scholar] [CrossRef]
- Onuma, Y.; Serruys, P. Bioresorbable Scaffold. Circulation 2011, 123, 779–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, J.; Onuma, Y.; Ormiston, J.; Abizaid, A.; Waksman, R.; Serruys, P. Bioresorbable scaffolds: Rationale, current status, challenges, and future. Eur. Heart J. 2013, 35, 765–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ormiston, J.A.; Serruys, P.W.; Onuma, Y.; Van Geuns, R.-J.; De Bruyne, B.; Dudek, D.; Thuesen, L.; Smits, P.C.; Chevalier, B.; McClean, D.; et al. First Serial Assessment at 6 Months and 2 Years of the Second Generation of Absorb Everolimus-Eluting Bioresorbable Vascular Scaffold. Circ. Cardiovasc. Interv. 2012, 5, 620–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serruys, P.W.; Ormiston, J.; van Geuns, R.-J.; de Bruyne, B.; Dudek, D.; Christiansen, E.; Chevalier, B.; Smits, P.; McClean, D.; Koolen, J.; et al. A Polylactide Bioresorbable Scaffold Eluting Everolimus for Treatment of Coronary Stenosis. J. Am. Coll. Cardiol. 2016, 67, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Stefanini, G.G.; Capodanno, D.; Onuma, Y.; Baumbach, A.; Escaned, J.; Haude, M.; James, S.; Joner, M.; Jüni, P.; et al. Report of an ESC-EAPCI Task Force on the evaluation and use of bioresorbable scaffolds for percutaneous coronary intervention: Executive summary. Eur. Heart J. 2017, 39, 1591–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jinnouchi, H.; Torii, S.; Sakamoto, A.; Kolodgie, F.D.; Virmani, R.; Finn, A.V. Fully bioresorbable vascular scaffolds: Lessons learned and future directions. Nat. Rev. Cardiol. 2018, 16, 286–304. [Google Scholar] [CrossRef]
- Kolandaivelu, K.; Swaminathan, R.; Gibson, W.J.; Kolachalama, V.B.; Nguyen-Ehrenreich, K.-L.; Giddings, V.L.; Coleman, L.; Wong, G.K.; Edelman, E. Stent Thrombogenicity Early in High-Risk Interventional Settings Is Driven by Stent Design and Deployment and Protected by Polymer-Drug Coatings. Circulation 2011, 123, 1400–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, C.; Palmaz, J.C.; Sprague, E. Influence of Topography on Endothelialization of Stents: Clues for New Designs. J. Autom. Inf. Sci. 2000, 10, 143–151. [Google Scholar] [CrossRef]
- Shibata, M.; Teramoto, N.; Inoue, Y. Mechanical properties, morphologies, and crystallization behavior of plasticized poly (l-lactide)/poly (butylene succinate-co-l-lactate) blends. Polymer 2007, 48, 2768–2777. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Y.; Zhang, M.; Zhou, W. Phase behavior and its viscoelastic response of polylactide/poly(ε-caprolactone) blend. Eur. Polym. J. 2008, 44, 2171–2183. [Google Scholar] [CrossRef]
- Sanusi, O.M.; Benelfellah, A.; Bikiaris, D.N.; Hocine, N.A. Effect of rigid nanoparticles and preparation techniques on the performances of poly (lactic acid) nanocomposites: A review. Polym. Adv. Technol. 2021, 32, 444–460. [Google Scholar] [CrossRef]
- Raquez, J.-M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Blair, R.; Dunne, N.; Lennon, A.; Menary, G. Processing-property relationships of biaxially stretched poly (L-lactic acid) sheet for application in coronary stents. J. Mech. Behav. Biomed. Mater. 2018, 86, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Dillon, B.; Doran, P.; Fuenmayor, E.; Healy, A.V.; Gately, N.M.; Major, I.; Lyons, J.G. Influence of Annealing and Biaxial Expansion on the Properties of Poly (l-Lactic Acid) Medical Tubing. Polymer 2019, 11, 1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Androsch, R.; Schick, C.; Di Lorenzo, M.L. Kinetics of Nucleation and Growth of Crystals of Poly (l-lactic acid). In Advances in Polymer Science; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2017; Volume 279, pp. 235–272. [Google Scholar]
- Ramachandran, K. Bioresorbable Vascular Scaffolds Gain Ductility, Resistance to Hydrolysis, and Radial Strength via a Unique Poly L-Lactide Microstructure. Ph.D. Thesis, California Institute of Technology, Pasadena, CA, USA, 27 November 2019. [Google Scholar]
- Bojda, J.; Piorkowska, E. Shear-induced nonisothermal crystallization of two grades of PLA. Polym. Test. 2016, 50, 172–181. [Google Scholar] [CrossRef]
- Iqbal, N.; Jariyavidyanont, K.; Rhoades, A.M.; Androsch, R. Critical specific work of flow for shear-induced formation of crystal nuclei in poly (l -lactic acid). Polym. Cryst. 2019, 2, 7. [Google Scholar] [CrossRef]
- Zhong, Y.; Fang, H.; Zhang, Y.; Wang, Z.; Yang, J.; Wang, Z. Rheologically Determined Critical Shear Rates for Shear-Induced Nucleation Rate Enhancements of Poly (lactic acid). ACS Sustain. Chem. Eng. 2013, 1, 663–672. [Google Scholar] [CrossRef]
- Xu, H.; Xie, L.; Chen, Y.-H.; Huang, H.-D.; Xu, J.-Z.; Zhong, G.-J.; Hsiao, B.S.; Li, Z.-M. Strong Shear Flow-Driven Simultaneous Formation of Classic Shish-Kebab, Hybrid Shish-Kebab, and Transcrystallinity in Poly (lactic acid)/Natural Fiber Biocomposites. ACS Sustain. Chem. Eng. 2013, 1, 1619–1629. [Google Scholar] [CrossRef]
- Yang, J.; Wang, C.; Wang, K.; Zhang, Q.; Chen, F.; Du, R.; Fu, Q. Direct Formation of Nanohybrid Shish-Kebab in the Injection Molded Bar of Polyethylene/Multiwalled Carbon Nanotubes Composite. Macromolecules 2009, 42, 7016–7023. [Google Scholar] [CrossRef]
- Ramachandran, K.; Miscioscia, R.; De Filippo, G.; Pandolfi, G.; Di Luccio, T.; Kornfield, J.A. Tube Expansion Deformation Enables in Situ Synchrotron X-ray Scattering Measurements during Extensional Flow-Induced Crystallization of Poly l-Lactide Near the Glass Transition. Polymer 2018, 10, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mai, F.; Wang, K.; Yao, M.; Deng, H.; Chen, F.; Fu, Q. Superior Reinforcement in Melt-Spun Polyethylene/Multiwalled Carbon Nanotube Fiber through Formation of a Shish-Kebab Structure. J. Phys. Chem. B 2010, 114, 10693–10702. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Cheng, Z.; Yang, S.; Sun, X.; Xie, L.; Zheng, Q. Extensional flow-induced conductive nanohybrid shish in poly (lactic acid) nanocomposites toward pioneering combination of high electrical conductivity, strength, and ductility. Compos. Part B Eng. 2021, 207, 108556. [Google Scholar] [CrossRef]
- Zhou, M.; Fan, M.; Zhao, Y.; Jin, T.; Fu, Q. Effect of stretching on the mechanical properties in melt-spun poly (butylene succinate)/microfibrillated cellulose (MFC) nanocomposites. Carbohydr. Polym. 2016, 140, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhong, G.-J.; Fu, Q.; Lei, J.; Jiang, W.; Hsiao, B.S.; Li, Z.-M. Formation of Shish-Kebabs in Injection-Molded Poly (l-lactic acid) by Application of an Intense Flow Field. ACS Appl. Mater. Interfaces 2012, 4, 6774–6784. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Wang, Y.; Shen, C.; Liu, C.; Wang, Z. Melt extension-induced shish-kebabs with heterogeneous spatial distribution of crystalline polymorphs in lightly crosslinked poly (lactic acid). Polymer 2020, 208, 122875. [Google Scholar] [CrossRef]
- Tang, H.; Chen, J.-B.; Wang, Y.; Xu, J.-Z.; Hsiao, B.S.; Zhong, G.-J.; Li, Z.-M. Shear Flow and Carbon Nanotubes Synergistically Induced Nonisothermal Crystallization of Poly (lactic acid) and Its Application in Injection Molding. Biomacromolecules 2012, 13, 3858–3867. [Google Scholar] [CrossRef] [PubMed]
- Løvdal, A.; Andreasen, J.W.; Mikkelsen, L.P.; Agersted, K.; Almdal, K. Characterization of biaxial strain of poly (l -lactide) tubes. Polym. Int. 2015, 65, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, K.; Di Luccio, T.; Ailianou, A.; Kossuth, M.B.; Oberhauser, J.P.; Kornfield, J.A. Crimping-induced structural gradients explain the lasting strength of poly l-lactide bioresorbable vascular scaffolds during hydrolysis. Proc. Natl. Acad. Sci. USA 2018, 115, 10239–10244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Żak, A.; Sallacan-Ecker, L.; Margolin, A.; Feldman, Y.; Popovitz-Biro, R.; Albu-Yaron, A.; Genut, M.; Tenne, R. Scaling Up of the WS2Nanotubes Synthesis. Full Nanotub. Carbon Nanostruct. 2010, 19, 18–26. [Google Scholar] [CrossRef]
- Tenne, R.; Margulis, L.; Genut, M.; Hodes, G. Polyhedral and cylindrical structures of tungsten disulphide. Nat. Cell Biol. 1992, 360, 444–446. [Google Scholar] [CrossRef]
- Kaplan-Ashiri, I.; Cohen, S.R.; Gartsman, K.; Ivanovskaya, V.; Heine, T.; Seifert, G.; Wiesel, I.; Wagner, H.D.; Tenne, R. On the mechanical behavior of WS2 nanotubes under axial tension and compression. Proc. Natl. Acad. Sci. USA 2006, 103, 523–528. [Google Scholar] [CrossRef] [Green Version]
- Appel, J.H.; Li, D.O.; Podlevsky, J.D.; Debnath, A.; Green, A.; Wang, Q.H.; Chae, J. Low Cytotoxicity and Genotoxicity of Two-Dimensional MoS2 and WS2. ACS Biomater. Sci. Eng. 2016, 2, 361–367. [Google Scholar] [CrossRef]
- Goldman, E.B.; Zak, A.; Tenne, R.; Kartvelishvily, E.; Levin-Zaidman, S.; Neumann, Y.; Stiubea-Cohen, R.; Palmon, A.; Hovav, A.-H.; Aframian, D.J. Biocompatibility of Tungsten Disulfide Inorganic Nanotubes and Fullerene-Like Nanoparticles with Salivary Gland Cells. Tissue Eng. Part A 2015, 21, 1013–1023. [Google Scholar] [CrossRef]
- Pardo, M.; Shuster-Meiseles, T.; Levin-Zaidman, S.; Rudich, A.; Rudich, Y. Low Cytotoxicity of Inorganic Nanotubes and Fullerene-Like Nanostructures in Human Bronchial Epithelial Cells: Relation to Inflammatory Gene Induction and Antioxidant Response. Environ. Sci. Technol. 2014, 48, 3457–3466. [Google Scholar] [CrossRef]
- Song, P.; Sang, L.; Zheng, L.; Wang, C.; Liu, K.; Wei, Z. Insight into the role of bound water of a nucleating agent in polymer nucleation: A comparative study of anhydrous and monohydrated orotic acid on crystallization of poly (l-lactic acid). RSC Adv. 2017, 7, 27150–27161. [Google Scholar] [CrossRef] [Green Version]
- Shalom, H.; Kapishnikov, S.; Brumfeld, V.; Naveh, N.; Tenne, R.; Lachman, N. Strong, tough and bio-degradable polymer-based 3D-ink for fused filament fabrication (FFF) using WS2 nanotubes. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Shalom, H.; Sui, X.; Elianov, O.; Brumfeld, V.; Rosentsveig, R.; Pinkas, I.; Feldman, Y.; Kampf, N.; Wagner, H.; Lachman, N.; et al. Nanocomposite of Poly (l-Lactic Acid) with Inorganic Nanotubes of WS2. Lubricants 2019, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Loffredo, F.; Tammaro, L.; Di Luccio, T.; Borriello, C.; Villani, F.; De Vito, S.; Ramachandran, K.; Kornfield, J.A. Effect of tungsten disulfide nanotubes on crystallization of polylactide under uniaxial deformation and annealing. Funct. Compos. Mater. 2021, 2, 1–11. [Google Scholar] [CrossRef]
- Garcia-Garcia, H.M.; Serruys, P.W.; Campos, C.M.; Muramatsu, T.; Nakatani, S.; Zhang, Y.-J.; Onuma, Y.; Stone, G.W. Assessing Bioresorbable Coronary Devices. JACC Cardiovasc. Imaging 2014, 7, 1130–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chithaiah, P.; Ghosh, S.; Idelevich, A.; Rovinsky, L.; Livneh, T.; Zak, A. Solving the “MoS2 Nanotubes” Synthetic Enigma and Elucidating the Route for Their Catalyst-Free and Scalable Production. ACS Nano 2020, 14, 3004–3016. [Google Scholar] [CrossRef] [PubMed]
- Glauser, T.; Gueriguian, V.; Steichen, B.; Oberhauser, J.; Gada, M.; Kleiner, L.; Kossuth, M.B. Controlling Crystalline Morphology of Bioabsorbable Stent. U.S. Patent 9.211.682, 15 December 2015. [Google Scholar]
- Di Lorenzo, M.L. Crystallization behavior of poly (l-lactic acid). Eur. Polym. J. 2005, 41, 569–575. [Google Scholar] [CrossRef]
- Huirache-Acuña, R.; Paraguay-Delgado, F.; Albiter, M.A.; Alvarez-Contreras, L.; Rivera-Muñoz, E.M.; Alonso-Núñez, G. Synthesis and characterization of WO3 and WS2 hexagonal phase nanostructures and catalytic test in sulfur remotion. J. Mater. Sci. 2009, 44, 4360–4369. [Google Scholar] [CrossRef]
- Yue, H.; Reguero, V.; Senokos, E.; Monreal-Bernal, A.; Mas, B.; Fernández-Blázquez, J.; Marcilla, R.; Vilatela, J. Fractal carbon nanotube fibers with mesoporous crystalline structure. Carbon 2017, 122, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Yue, H.; Monreal-Bernal, A.; Fernández-Blázquez, J.P.; Llorca, J.; Vilatela, J.J. Macroscopic CNT fibres inducing non-epitaxial nucleation and orientation of semicrystalline polymers. Sci. Rep. 2015, 5, 16729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffery, G.B. The motion of ellipsoidal particles immersed in a viscous fluid. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1922, 102, 161–179. [Google Scholar] [CrossRef] [Green Version]
- Bretherton, F.P. The motion of rigid particles in a shear flow at low Reynolds number. J. Fluid Mech. 1962, 14, 284–304. [Google Scholar] [CrossRef]
- Abbott. Available online: https://abbott.mediaroom.com/2020-09-03-Abbott-Announces-Start-of-Trial-to-Evaluate-the-New-Esprit-TM-BTK-Drug-Eluting-Resorbable-Scaffold (accessed on 14 May 2020).
- REVA. Medical. Available online: https://www.revamedical.com/wp-content/uploads/2018/08/tyrocore-technical-summary_cc100056reva-2.pdf (accessed on 14 May 2020).








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocher, L.; Ylitalo, A.S.; Di Luccio, T.; Miscioscia, R.; De Filippo, G.; Pandolfi, G.; Villani, F.; Zak, A.; Menary, G.H.; Lennon, A.B.; et al. Interaction of Poly L-Lactide and Tungsten Disulfide Nanotubes Studied by In Situ X-ray Scattering during Expansion of PLLA/WS2NT Nanocomposite Tubes. Polymers 2021, 13, 1764. https://doi.org/10.3390/polym13111764
Rocher L, Ylitalo AS, Di Luccio T, Miscioscia R, De Filippo G, Pandolfi G, Villani F, Zak A, Menary GH, Lennon AB, et al. Interaction of Poly L-Lactide and Tungsten Disulfide Nanotubes Studied by In Situ X-ray Scattering during Expansion of PLLA/WS2NT Nanocomposite Tubes. Polymers. 2021; 13(11):1764. https://doi.org/10.3390/polym13111764
Chicago/Turabian StyleRocher, Lison, Andrew S. Ylitalo, Tiziana Di Luccio, Riccardo Miscioscia, Giovanni De Filippo, Giuseppe Pandolfi, Fulvia Villani, Alla Zak, Gary H. Menary, Alex B. Lennon, and et al. 2021. "Interaction of Poly L-Lactide and Tungsten Disulfide Nanotubes Studied by In Situ X-ray Scattering during Expansion of PLLA/WS2NT Nanocomposite Tubes" Polymers 13, no. 11: 1764. https://doi.org/10.3390/polym13111764
APA StyleRocher, L., Ylitalo, A. S., Di Luccio, T., Miscioscia, R., De Filippo, G., Pandolfi, G., Villani, F., Zak, A., Menary, G. H., Lennon, A. B., & Kornfield, J. A. (2021). Interaction of Poly L-Lactide and Tungsten Disulfide Nanotubes Studied by In Situ X-ray Scattering during Expansion of PLLA/WS2NT Nanocomposite Tubes. Polymers, 13(11), 1764. https://doi.org/10.3390/polym13111764