Investigation of Polymer Biofilm Formation on Titanium-Based Anode Surface in Microbial Fuel Cells with Poplar Substrate
Abstract
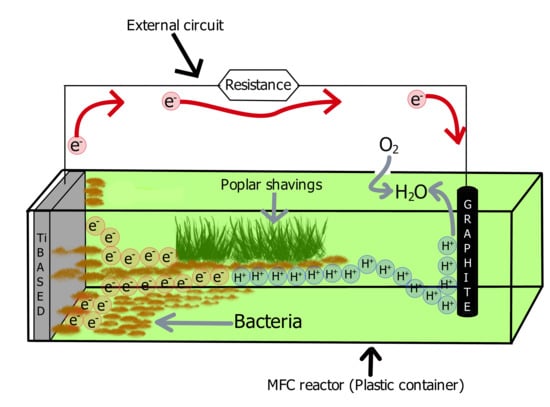
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
3.1. Electrochemical Performance of MFCs
3.2. Fourier Transform Infrared Spectroscopy Analysis of Poplar Wood Shavings and Electrodes
3.3. Scanning Electron Microscopy and Energy-Dispersive Spectrometer Analysis
3.4. Bacteria Analyses and Biofilm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Çek, N. Examination of Zinc Electrode Performance in Microbial Fuel Cells. Gazi Univ. J. Sci. 2017, 30, 395–402. [Google Scholar]
- Erensoy, A.; Çek, N. Alternative Biofuel Materials for Microbial Fuel Cells from Poplar Wood. ChemistrySelect 2018, 3, 11251–11257. [Google Scholar] [CrossRef]
- Çek, N.; Erensoy, A. Yakıt Hücresi Teknolojilerinde Gelişmeler, 1st ed.; Nobel Academic Publishing: Ankara, Turkey, 2020; pp. 137–226. [Google Scholar]
- Çek, N. Parçacıklar ve Enerji Kaynakları, 1st ed.; Lambert Academic Publishing: Saarbrucken, Germany, 2016; pp. 157–177. [Google Scholar]
- Sasaki, D.; Sasaki, K.; Tsuge, Y.; Kondo, A. Less biomass and intracellular glutamate in anodic biofilms lead to efficient electricity generation by microbial fuel cells. Biotechnol. Biofuels 2019, 12, 72. [Google Scholar] [CrossRef]
- Angelaalincy, M.J.; Navanietha Krishnaraj, R.; Shakambari, G.; Ashokkumar, B.; Kathiresan, S.; Varalakshmi, P. Biofilm Engineering Approaches for Improving the Performance of Microbial Fuel Cells and Bioelectrochemical Systems. Front. Energy Res. 2018, 6, 63. [Google Scholar] [CrossRef]
- Stöckl, M.; Teubner, N.C.; Holtmann, D.; Mangold, K.-M.; Sand, W. Extracellular Polymeric Substances from Geobacter sulfurreducens Biofilms in Microbial Fuel Cells. ACS Appl. Mater. Interfaces 2019, 11, 8961–8968. [Google Scholar] [CrossRef]
- You, J.; Walter, X.A.; Greenman, J.; Melhuish, C.; Ieropoulos, I. Stability and reliability of anodic biofilms under different feedstock conditions: Towards microbial fuel cell sensors. Sens. Bio-Sens. Res. 2015, 6, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Devappa, R.K.; Rakshit, S.K.; Dekker, R.F.H. Forest biorefinery: Potential of poplar phytochemicals as value-added co-products. Biotechnol. Adv. 2015, 33, 681–716. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Bian, H.; Wei, L.; Wnag, W. Enhancement of Hydrotropic Fractionation of Poplar Wood Using Autohydrolysis and Disk Refining Pretreatment: Morphology and Overall Chemical Characterization. Polymers 2019, 11, 685. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Kim, J.R.; Heo, J. Enhancement of bioelectricity generation by a microbial fuel cell using Ti nanoparticle-modified carbon electrode. J. Chem. Technol. Biotechnol. 2019, 94, 1622–1627. [Google Scholar] [CrossRef]
- Magotra, V.K.; Kumar, S.; Kang, T.W.; Inamdar, A.I.; Aqueel, A.T.; Im, H.; Ghodake, G.; Shinde, S.; Waghmode, D.P.; Jeon, H.C. Compost Soil Microbial Fuel Cell to Generate Power using Urea as Fuel. Sci. Rep. 2020, 10, 4154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.-B.; Zhong, W.-H.; Han, C.; Deng, H. Characterization of Electricity Generated by Soil in Microbial Fuel Cells and the Isolation of Soil Source Exoelectrogenic Bacteria. Front. Microbiol. 2016, 7, 1776. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, Y.; Pedrycz, W.; Guo, Y.A. Terrestrial Microbial Fuel Cell for Powering a Single-Hop Wireless Sensor Network. Int. J. Mol. Sci. 2016, 17, 762. [Google Scholar] [CrossRef] [PubMed]
- Simeon, M.I.; Asoiro, F.U.; Aliyu, M.; Raji, O.A.; Freitag, R. Polarization and power density trends of a soil-based microbial fuel cell treated with human urine. Int. J. Energy Res. 2020, 44, 5968–5976. [Google Scholar] [CrossRef]
- Helder, M.; Strik, D.P.; Hamelers, H.V.; Buisman, C.J.N. The flat-plate plant-microbial fuel cell: The effect of a new design on internal resistances. Biotechnol. Biofuels 2012, 5, 70. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Xing, S.; Lia, J. FTIR Studies of the Changes in Wood Chemistry from Wood Forming Tissue under Inclined Treatment. Energy Procedia 2012, 16, 758–762. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Liu, Z.; Hui, L.; Ma, L.; Zheng, X.; Li, J.; Zhang, Y. Understanding the structural changes of lignin in poplar following steam explosion pretreatment. Holzforschung 2020, 74, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, J.; Li, M.; Pu, Y.; Ragauskas, A.J.; Yoo, C.G. Observation of Potential Contaminants in Processed Biomass Using Fourier Transform Infrared Spectroscopy. Appl. Sci. 2020, 10, 4345. [Google Scholar] [CrossRef]
- Mustafa, M.N.; Shafie, S.; Zainal, Z.; Sulaiman, Y. A Novel Poly(3,4-ethylenedioxythiophene)-graphene Oxide/Titanium Dioxide Composites Counter Electrode for Dye-Sensitized Solar Cell. J. Nanomater. 2017, 4045672. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, P.; Uday, U.S.P.; Bandyopadhyay, T.K.; Ray, N.R.; Bhunia, B. Performance improvement of microbial fuel cell (MFC) using suitable electrode and Bioengineered organisms: A review. Bioengineered 2017, 8, 471–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Read, S.T.; Dutta, P.; Bond, P.L.; Keller, J.; Rabaey, K. Initial development and structure of biofilms on microbial fuel cell anodes. BMC Microbiol. 2010, 10, 98. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhu, X.; Li, J.; Liao, Q.; Ye, D. Biofilm formation and electricity generation of a microbial fuel cell started up under different external resistances. J. Power Sources 2011, 196, 6029–6035. [Google Scholar] [CrossRef]








| Element (Weight %) | Ti-Based Anode | P1-MFC | P2-MFC | P3-MFC |
|---|---|---|---|---|
| Titanium | 87.40 | 75.20 | 62.50 | 51.48 |
| Oxygen | 12.60 | 18.42 | 25.61 | 33.99 |
| Carbon | 0.0 | 6.38 | 11.89 | 14.53 |
| Wavenumber (cm−1) | Assignment | Components |
|---|---|---|
| 3367 | O–H stretching | cellulose, hemicellulose, lignin |
| 2914 | C–H stretching | cellulose, hemicellulose, lignin |
| 1745 | C=O stretching | hemicellulose, lignin |
| 1618 | Aromatic skeletal vibration, C=O stretching, adsorbed O–H | hemicellulose, lignin |
| 1508 | C=C–C aromatic ring stretching and vibration | lignin |
| 1457 | C–H deformation (in methyl and methylene) | lignin |
| 1424 | Symmetric CH2 bending vibration, symmetric stretching band of carboxyl group, C–H deformation | cellulose, hemicellulose, lignin |
| 1370 | C–H bending, C–H stretching in CH3 | cellulose, hemicellulose, lignin |
| 1317 | CH2 wagging, C–O stretching of C5 substituted aromatic units | cellulose, hemicellulose, lignin |
| 1235 | C–O stretching of guaiacyl unit | lignin |
| 1160 | C–O–C stretching | cellulose, hemicellulose |
| 1108 | Aromatic C–H in plane deformation | lignin |
| 1053 | C–OH stretching vibration, C–O deformation | cellulose, hemicellulose, lignin |
| 1032 | C–O stretching, aromatic C–H in plane deformation | cellulose, lignin |
| 896 | C–O–C stretching | cellulose, hemicellulose |
| 846 | Aromatic C–H out of plane bending | lignin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erensoy, A.; Çek, N. Investigation of Polymer Biofilm Formation on Titanium-Based Anode Surface in Microbial Fuel Cells with Poplar Substrate. Polymers 2021, 13, 1833. https://doi.org/10.3390/polym13111833
Erensoy A, Çek N. Investigation of Polymer Biofilm Formation on Titanium-Based Anode Surface in Microbial Fuel Cells with Poplar Substrate. Polymers. 2021; 13(11):1833. https://doi.org/10.3390/polym13111833
Chicago/Turabian StyleErensoy, Ahmet, and Nurettin Çek. 2021. "Investigation of Polymer Biofilm Formation on Titanium-Based Anode Surface in Microbial Fuel Cells with Poplar Substrate" Polymers 13, no. 11: 1833. https://doi.org/10.3390/polym13111833
APA StyleErensoy, A., & Çek, N. (2021). Investigation of Polymer Biofilm Formation on Titanium-Based Anode Surface in Microbial Fuel Cells with Poplar Substrate. Polymers, 13(11), 1833. https://doi.org/10.3390/polym13111833