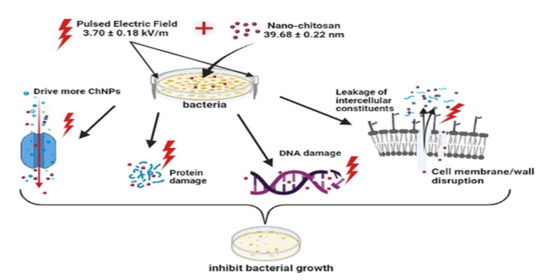
Enhancement of Nano-Biopolymer Antibacterial Activity by Pulsed Electric Fields
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electric Field Exposure System
2.2. Nano-Chitosan Characterizations
2.3. Bacterial Strains and Replications
2.4. Bacterial Growth Characteristics and Antibacterial Impacts
2.5. Bacterial Cytotoxicity Tests
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kiroshka, V.V.; Petrova, V.A.; Chernyakov, D.D.; Bozhkova, Y.O.; Kiroshka, K.V.; Baklagina, Y.G.; Romanov, D.P.; Kremnev, R.V.; Skorik, Y.A. Influence of chitosan-chitin nanofiber composites on cytoskeleton structure and the proliferation of rat bone marrow stromal cells. J. Mater. Sci. Mater. Med. 2017, 28, 21. [Google Scholar] [CrossRef]
- Petrova, V.A.; Elokhovskiy, V.Y.; Raik, S.V.; Poshina, D.N.; Romanov, D.P.; Skorik, Y.A. Alginate gel reinforcement with chitin nanowhiskers modulates rheological properties and drug release profile. Biomolecules 2019, 9, 291. [Google Scholar] [CrossRef] [Green Version]
- Sarin, S.; Kolesnikova, S.; Postnova, I.; Ha, C.S.; Shchipunov, Y. Bionanocomposite from self-assembled building blocks of nacre-like crystalline polymorph of chitosan with clay nanoplatelets. RSC Adv. 2016, 6, 33501–33509. [Google Scholar] [CrossRef]
- Sun, F.; Pang, X.; Zhitomirsky, I. Electrophoretic deposition of composite hydroxyapatite–chitosan–heparin coatings. J. Mater. Process. Technol. 2009, 209, 1597–1606. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Kim, K.D.; Chun, S.C. Antibacterial Activity of Chitosan Nanoparticles: A Review. Processes 2020, 8, 1173. [Google Scholar] [CrossRef]
- Raafat, D.; Von Bargen, K.; Haas, A.; Sahl, H.-G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, C.; Alcock, E.; Buttimer, F.; Schmidt, M.; Clarke, D.; Pemble, M.; Bardosova, M. Synthesis and characterisation of cross-linked chitosan composites functionalised with silver and gold nanoparticles for antimicrobial applications. Sci. Technol. Adv. Mater. 2017, 18, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Susilowati, E. Green synthesis of silver-chitosan nanocomposite and their application as antibacterial material. J. Phys. Conf. Ser. 2019, 1153, 012135. [Google Scholar] [CrossRef]
- Małgorzata, Z.; Anna, D.; Yury, A.; Valentina, A.; Adam, C.; Iwona, K. Silver Nanoparticles on Chitosan/Silica Nanofibers: Characterization and Antibacterial Activity. Int. J. Mol. Sci. 2020, 21, 166. [Google Scholar]
- Yadav, A.V.; Bhise, B. Chitosan: A potencial biomaterial effective against typhoid. Curr. Sci. 2004, 87, 1176–1178. [Google Scholar]
- Zazouli, M.A.; Yousefi, M.; Kor, Y.; Roohafzaee, M. Inactivation of Escherichia coli in Water by Combined Process of Silver Nanoparticle and Ultraviolet Radiation. Health Scope 2016. [Google Scholar] [CrossRef]
- Fojt, L.; Strasak, L.; Vetterl, V.; Smarda, J. Comparison of the low-frequency magnetic field effects on bacteria Escherichia coli, Leclercia adecarboxylata and Staphylococcus aureus. Bioelectrochemistry 2004, 63, 337–341. [Google Scholar] [CrossRef]
- Inhan-Garip, A.; Aksu, B.; Akan, Z.; Akakin, D.; Ozaydin, A.N.; San, T. Effect of extremely low frequency electromagnetic fields on growth rate and morphology of bacteria. Int. J. Radiat. Biol. 2011, 87, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Cellini, L.; Grande, R.; Di Campli, E.; Di Bartolomeo, S.; Di Giulio, M.; Robuffo, I.; Trubiani, O.; Mariggiò, M.A. Bacterial response to the exposure of 50 Hz electromagnetic fields. Bioelectromagnetics 2008, 29, 302–311. [Google Scholar] [CrossRef]
- Belyaev, I. Toxicity and SOS-response to ELF magnetic fields and nalidixic acid in E. coli cells. Mutat. Res. 2011, 722, 56–61. [Google Scholar] [CrossRef]
- Segatore, B.; Setacci, D.; Bennato, F.; Cardigno, R.; Amicosante, G.; Iorio, R. Evaluations of the Effects of Extremely Low-Frequency Electromagnetic Fields on Growth and Antibiotic Susceptibility of Escherichia coli and Pseudomonas aeruginosa. Int. J. Microbiol. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [Green Version]
- El-Kaliuoby, M.I.; El-Khatib, A.M.; Khalil, A.M. Does Engineering of Nano Shapes Have Antibacterial Synergy with Magnetic Signals Exposure? Surf. Innov. 2019, 7, 260–269. [Google Scholar] [CrossRef]
- El-Khatib, A.M.; Khalil, A.M.; El-Kaliuoby, M.I.; El-Khatib, M. The Combined Effects of Multi-Sized Silver Nanoparticles by Arc Discharge and Pulsed Magnetic Fields Exposure on K. pneumoniae. Bioinspir. Biomim. Nanobiomater. 2019, 8, 154–160. [Google Scholar] [CrossRef]
- El-kaliuoby, M.I.; Khalil, A.M.; El-Khatib, A.M.; Shalaby, T.I. Synergistic antibacterial effect of silver nanoparticles and extremely low frequency pulsed magnetic fields on Klebsiella pneumoniae. J. Appl. Biol. Biotech. 2018, 6, 039–045. [Google Scholar]
- El-Kaliuoby, M.I.; Khalil, A.M.; El-Khatib, A.M.; Shehata, N. Antibacterial Synergism of Electrospun Nanofiber Mats Functioned with Silver Nanoparticles and Pulsed Electromagnetic Waves. Polymers 2021, 13, 277. [Google Scholar] [CrossRef] [PubMed]
- El-kaliuoby, M.I.; Hamouda, R.; El-Khatib, A.M. Novel approach to enhancing remineralization of tooth surface by magnetic field exposure. Surf. Innov. 2020, 9, 49–56. [Google Scholar] [CrossRef]
- Seeliger, C.; Falldorf, K.; Sachtleben, J.; Griensven, M. Low-frequency pulsed electromagnetic fields significantly improve time of closure and proliferation of human tendon fibroblasts. Eur. J. Med. Res. 2014, 19, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirogova, E.; Vojisavljevic, V.; Ahmed, I. Investigation of the effect of Extremely Low Frequency (ELF) Pulsed Electromagnetic Field (PEMF) on Collagenase Enzyme Kinetics. In Proceedings of the 6th International Joint Conference on Biomedical Engineering Systems and Technologies INSTICC, Barcelona, Spain, 11–14 February 2013; BIODEVICES 2013: Lisbon, Portugal, 2013; pp. 143–147. [Google Scholar]
- Jamil, M.M.A.; Zaltum, M.A.; Youseffi, M.; Javid, F. Study on Pulse Electric Field Exposure Effect on HeLa Cells For Wound Healing Application. International Conference on Biomedical Engineering (ICoBE). J. Phys. Conf. Ser. 2019, 1372, 012021. [Google Scholar] [CrossRef]
- Rivera Aguayo, P.; Bruna Larenas, T.; Alarcón Godoy, C.; Cayupe Rivas, B.; González-Casanova, J.; Rojas-Gómez, D.; Caro Fuentes, N. Antimicrobial and Antibiofilm Capacity of Chitosan Nanoparticles against Wild Type Strain of Pseudomonas sp. Isolated from Milk of Cows Diagnosed with Bovine Mastitis. Antibiotics 2020, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Golberg, A.; Broelsch, G.F.; Bohr, S.; Mihm, M.C.; Austen, W.G.; Albadawi, H.; Yarmush, M.L. Non-thermal, pulsed electric field cell ablation: A novel tool for regenerative medicine and scarless skin regeneration. Technology 2013, 01, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Golberg, A.; Khan, S.; Belov, V.; Quinn, K.P.; Albadawi, H.; Felix Broelsch, G.; Yarmush, M.L. Skin Rejuvenation with Non-Invasive Pulsed Electric Fields. Sci. Rep. 2015, 5, 10187. [Google Scholar] [CrossRef] [Green Version]
- Amin, A.N.; Cerceo, E.A.; Deitelzweig, S.B.; Pile, J.C.; Rosenberg, D.J.; Sherman, B.M. Hospitalist perspective on the treatment of skin and soft tissue infections. Mayo Clin. Proc. 2014, 89, 1436–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjarnsholt, T.; Kirketerp-Møller, K.; Jensen, P.Ø.; Madsen, K.G.; Phipps, R.; Krogfelt, K.; Høiby, N.; Givskov, M. Why chronic wounds will not heal: A novel hypothesis. Wound Rep. Regen. 2008, 16, 2–10. [Google Scholar] [CrossRef]
- Schaber, J.A.; Triffo, W.J.; Suh, S.J.; Oliver, J.W.; Hastert, M.C.; Griswold, J.A.; Auer, M.; Hamood, A.N.; Rumbaugh, K.P. Pseudomonas aeruginosa Forms Biofilms in Acute Infection Independent of Cell-to-Cell Signaling. Infect. Immun. 2007, 75, 3715–3721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn Wound Infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef] [Green Version]
- Hempel, K.; Pané-Farré, J.; Otto, A.; Sievers, S.; Hecker, M.; Becher, D. Quantitative Cell Surface Proteome Profiling for SigB-Dependent Protein Expression in the Human Pathogen Staphylococcus aureusvia Biotinylation Approach. J. Proteome Res. 2010, 9, 1579–1590. [Google Scholar] [CrossRef]
- Abeyrathne, C.D.; Huynh, D.H.; Mcintire, T.W.; Nguyen, T.C.; Nasr, B.; Zantomio, D.; Chana, G.; Abbott, I.; Choong, P.; Catton, M.; et al. Lab on a chip sensor for rapid detection and antibiotic resistance determination of Staphylococcus aureus. Analyst 2016, 141, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Elmi, F.; Alinezhad, H.; Moulana, Z.; Salehian, F.; Mohseni Tavakkoli, S.; Asgharpour, F.; Fallah, H.; Elmi, M.M. The use of antibacterial activity of ZnO nanoparticles in the treatment of municipal wastewater. Water Sci. Technol. 2014, 70, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Fadel, M.; Ali, A.M.; Elkhatib, W.M.; Aboutalib, A.M.; Abdelbacki Khalil, A.M.; Serag, N. Control of the Activity of Pseudomonas Aeruginosa by Positive Electric Impulses at Resonance Frequency. J. Am. Sci. 2013, 9, 120–130. [Google Scholar]
- El-kaliuoby, M.I.; Khalil, A.M.; El-Khatib, A.M. Alterations of Bacterial Dielectric Characteristics Due to Pulsed Magnetic Field Exposures. Bioinspir. Biomim. Nanobiomater. 2020, 9, 103–111. [Google Scholar] [CrossRef]
- Dhermendra, K.; Behari, T.J.; Sen, P. Time and dose-dependent antimicrobial potential of Ag nanoparticles synthesized by top-down approach. Curr. Sci. 2008, 95, 647–655. [Google Scholar]
- Kim, S.H.; Lee, H.S.; Ryu, D.S.; Choi, S.J.; Lee, D.S. Antibacterial Activity of Silver-nanoparticles Against Staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 2011, 39, 77–85. [Google Scholar]
- Bradford, M.M. A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1967, 72, 248–254. [Google Scholar] [CrossRef]
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Zeng, H.Y.; Ou-Yang, Y.S.; Chen, Y.B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef]
- Reddy, L.S.; Nisha, M.M.; Joice, M.; Shilpa, P.N. Antimicrobial activity of zinc oxide (ZnO) nanoparticle against Klebsiella pneumoniae. Pharm. Biol. 2014, 52, 1388–1397. [Google Scholar] [CrossRef]
- Jia, Z.; Shen, D.; Xu, W. Synthesis and antibacterial activities of quaternary ammonium salt of chitosan. Carbohydr. Res. 2001, 333, 1–6. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, Y.; Liu, H. Preparation of new crosslinked chitosan with crown ether and their adsorption for silver ion for antibacterial activities. Carbohydr. Polym. 2003, 53, 425–430. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Birsoy, K.; Wang, T.C.; Chen, W.W.; Freinkman, E.; Abu-Remaileh, M.; Sabatini, D.M. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 2015, 162, 540–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, W.-L.; Niu, S.-S.; Xu, Y.-L.; Xu, Z.-R.; Fan, C.-L. Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr. Polym. 2009, 75, 385–389. [Google Scholar] [CrossRef]
- Joshi, M.; Ali, S.W.; Purwar, R. Ecofriendly antimicrobial finishing of textile using bioactive agents based on natural products. Indian J. Fibre Text. Res. 2009, 30, 295–304. [Google Scholar]
- Wang, X.; Du, Y.; Liu, H. Preparation, characterization and antimicrobial activity of chitosan–Zn complex. Carbohydr. Polym. 2004, 56, 21–26. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Su, Y.-P.; Chen, C.-C.; Jia, G.; Wang, H.-L.; Wu, J.C.G.; Lin, J.-G. Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol. Sin. 2004, 25, 932–936. [Google Scholar] [PubMed]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Divya, K.; Vijayan, S.; George, T.K.; Jisha, M.S. Antimicrobial properties of chitosan nanoparticles: Mode of action and factors affecting activity. Fibers Polym. 2017, 18, 221–230. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Lotfy, T.M.R.; Shawir, S.M.S. Preparation and antibacterial activity of chitosan-silver nanoparticles for application in preservation of minced meat. Bull. Natl. Res. Centre 2019, 43, 83. [Google Scholar] [CrossRef]
- Tran, H.V.; Tran, L.D.; Ba, C.T.; Vu, H.D.; Nguyen, T.N.; Pham, D.G.; Nguyen, P.X. Synthesis, characterization, antibacterial and antiproliferative activities of monodisperse chitosan-based silver nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 360, 32–40. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, J.; Saba, T.; Yue, Z.; Li, W.; Anderson, J.A.; Wang, X. Electric-Field-Assisted Facile Synthesis of Metal Nanoparticles. ACS Sustain. Chem. Eng. 2018, 7, 1271–1278. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Han, Z.; Zeng, X.; Yu, S.; Kennedy, J.F. Study on the degradation of chitosan by pulsed electric fields treatment. Innov. Food Sci. Emerg. Technol. 2010, 11, 587–591. [Google Scholar] [CrossRef]
- Dan, G.; Zhang, Z.H.; Zeng, X.A.; Han, Z.; Luo, W.B.; Tang, C.; Quek, S.Y. Synergetic Effects of Pulsed Electric Field and Ozone Treatments on the Degradation of High Molecular Weight Chitosan. Int. J. Food Eng. 2014, 10, 775–784. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhaus, G. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.-J.; Kim, H.; Liu, Y.; Han, H.-K.; Kwon, K.; Chang, K.-H.; Park, K.; Kim, Y.; Shim, K.; An, S.S.A.; et al. Incompatibility of silver nanoparticles with lactate dehydrogenase leakage assay for cellular viability test is attributed to protein binding and reactive oxygen species generation. Toxicol. Lett. 2014, 225, 422–432. [Google Scholar] [CrossRef]
- Yao, C.; Li, X.; Bi, W.; Jiang, C. Relationship between membrane damage, leakage of intracellular compounds, and inactivation of Escherichia coli treated by pressurized CO2. J. Basic Microbiol. 2013, 54, 858–865. [Google Scholar] [CrossRef]
- Guerriero, F.; Botarelli, E.; Mele, G.; Polo, L.; Zoncu, D.; Renati, P.; Sgarlata, C.; Rollone, M.; Ricevuti, G.; Maurizi, N.; et al. Effectiveness of an Innovative Pulsed Electromagnetic Fields Stimulation in Healing of Untreatable Skin Ulcers in the Frail Elderly: Two Case Reports. Case Rep. Dermatol. Med. 2015. [Google Scholar] [CrossRef] [Green Version]
- Ali, F.M.; Elkhatib, A.M.; Aboutaleb, W.M.; Abdelbacki, A.M.; Khalil, A.M.; El-kaliuoby, M.I. Control the Activity of Ralstonia Solanacearum Bacteria by Using Pulsed Electric Field. Jokull J. 2014, 64, 256–269. [Google Scholar]
- Balabel, N.M.; El-Kaliuoby, M.I.; Khalil, A.M. Effect of square pulsed magnetic field exposure on growth kinetics of Dickeya solani. Arch. Phytopathol. Plant Prot. 2019. [Google Scholar] [CrossRef]
- Goudarzi, I.; Hajizadeh, S.; Salmani, M.E.; Abrari, K. Pulsed electromagnetic fields accelerate wound healing in the skin of diabetic rats. Bioelectromagnetics 2010, 31, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.; Jalilifar, M. Electromagnetic Fields in the Treatment of Wound: A Review of Current Techniques and Future Perspective. J. Pure Appl. Microbiol. 2014, 8, 2863–2877. [Google Scholar]












| Conc. of ChNPs (mg/mL) | Count of P. aeruginosa (CFU/mL) | Count of S. aureus (CFU/mL) |
|---|---|---|
| 65.8 | NG | NG |
| 43.9 | NG | NG |
| 29.3 | NG | (7.77 ± 0.62) × 101 |
| 19.5 | NG | (4.79 ± 0.51) × 107 |
| 13.1 | (3.09 ± 0.13) × 101 | (2.91 ± 0.33) × 108 |
| 10.6 | (7.76 ± 0.18) × 103 | (8.51 ± 0.11) × 108 |
| 5.78 | (1.62 ± 0.26) × 106 | (1.28 ± 0.12) × 109 |
| 3.19 | (4.07 ± 0.43) × 107 | (2.81 ± 0.31) × 109 |
| 1.71 | (8.71 ± 0.35) × 108 | (5.37 ± 0.22) × 109 |
| Treatment Condition | Slope of Arbitrary Constants (µ) × 10−4 P. aeruginosa | Slope of Arbitrary Constants (µ) × 10−4 S. aureus |
|---|---|---|
| ChNPs | −(5.33 ± 0.86) | −(5.02 ± 0.56) |
| ChNPs +PEF | −(10.3 ± 0.96) | −(8.03 ± 0.75) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Kaliuoby, M.I.; Amer, M.; Shehata, N. Enhancement of Nano-Biopolymer Antibacterial Activity by Pulsed Electric Fields. Polymers 2021, 13, 1869. https://doi.org/10.3390/polym13111869
El-Kaliuoby MI, Amer M, Shehata N. Enhancement of Nano-Biopolymer Antibacterial Activity by Pulsed Electric Fields. Polymers. 2021; 13(11):1869. https://doi.org/10.3390/polym13111869
Chicago/Turabian StyleEl-Kaliuoby, Mai. I., Motaz Amer, and Nader Shehata. 2021. "Enhancement of Nano-Biopolymer Antibacterial Activity by Pulsed Electric Fields" Polymers 13, no. 11: 1869. https://doi.org/10.3390/polym13111869
APA StyleEl-Kaliuoby, M. I., Amer, M., & Shehata, N. (2021). Enhancement of Nano-Biopolymer Antibacterial Activity by Pulsed Electric Fields. Polymers, 13(11), 1869. https://doi.org/10.3390/polym13111869