Structure and Properties of Polyoxymethylene/Silver/Maleic Anhydride-Grafted Polyolefin Elastomer Ternary Nanocomposites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Ag Nanoparticles
2.3. Preparation of POM/Ag and POM/Ag/MAH-g-POENanocomposites
2.4. Injection Molding
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Scanning Electron Microscopy (SEM)
2.7. Thermogravimetric Analysis (TGA)
2.8. Differential Scanning Calorimetry (DSC)
2.9. Mechanical Testing
2.10. Dynamic Mechanical Analysis (DMA)
3. Results and Discussion
3.1. FTIRAnalysis
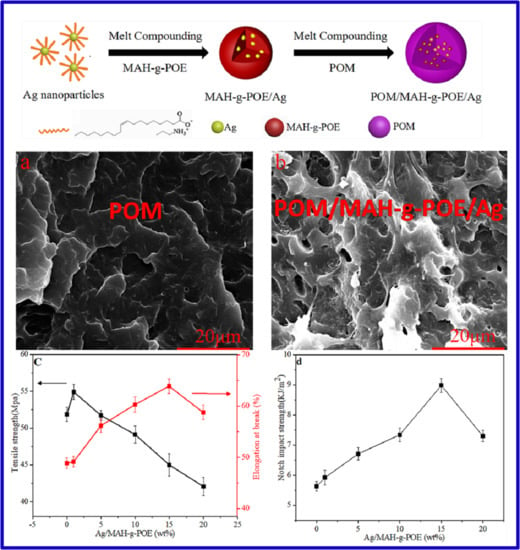
3.2. SEManalysis
3.3. TGAanalysis
3.4. DSC Analysis
3.5. Mechanical Properties
3.6. DMA Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Andrzejewski, J.; Skórczewska, K.; Kloziński, A. Improving the toughness and thermal resistance of polyoxymethylene/poly(lactic acid) blends: Evaluation of structure-properties correlation for reactive processing. Polymers 2020, 12, 307. [Google Scholar] [CrossRef] [Green Version]
- Berkowicz, G.; Majka, T.M.; Ukowski, W. The pyrolysis and combustion of polyoxymethylene in a fluid is ed bed with the possibility of incorporating CO2. Energy Convers. Manag. 2020, 214, 112888. [Google Scholar] [CrossRef]
- Bazan, P.; Kuciel, S.; Nykiel, M. Characterization of composites based on polyoxymethylene and effect of silicone addition on mechanical and tribological behavior. Polym. Eng. Sci. 2018, 59, 935–940. [Google Scholar] [CrossRef]
- Schrader, P.; Gosch, A.; Berer, M.; Marzi, S. Fracture of Thin-Walled Polyoxymethylene Bulk Specimens in Modes I and III. Materials 2020, 13, 5096. [Google Scholar] [CrossRef]
- Yang, J.; Yang, W.; Wang, X.; Dong, M.; Liu, H.; Wujcik, E.K.; Shao, Q.; Wu, S.; Ding, T.; Guo, Z. Synergistically toughening polyoxymethylene by methyl methacrylate-butadiene-styrene copolymer and thermoplastic polyurethane. Macro Mol. Chem. Phys. 2019, 220, 1800567. [Google Scholar] [CrossRef]
- Li, J.H.; Wang, Y.T.; Wang, X.D.; Wu, D.Z. Development of Polyoxymethylene/polylactide blends for a potentially biodegradable material: Crystallization kinetics, lifespan prediction, and enzymatic degradation behavior. Polymers 2019, 11, 1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Zhang, J.; Huang, J. Poly(lactic acid)/polyoxymethylene blends: Morphology, crystallization, rheology, and thermal mechanical properties. Polymer 2015, 69, 103–109. [Google Scholar] [CrossRef]
- Fang, X.; Wyatt, T.; Shi, J.; Yao, D. Fabrication of high-strength polyoxymethylene fibers by gel spinning. J. Mater. Sci. 2018, 53, 11901–11916. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, X.; Wang, H.; Wei, F.; Zhang, Y. Experimental and numerical analysis of interfacial bonding strength of polyoxymethylene reinforced cement composites. Constr. Build. Mater. 2019, 207, 1–9. [Google Scholar] [CrossRef]
- Jiao, Q.; Chen, Q.; Wang, L.; Chen, H.; Li, Y. Investigation on the crystallization behaviors of polyoxymethylene with a small amount of ionic liquid. Nanomaterials 2019, 9, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.C.; Tran, T.M.; Trinh, A.T.; Nguyen, A.H.; Dam, X.T.; Vu, Q.T.; Tran, D.L.; Nguyen, D.T.; Le, T.G.; Thai, H. Polyoxymethylene/silica/polylactic acid-grafted polyethylene glycol nanocomposites: Structure, morphology, and mechanical properties and ozone and UV durability. RSC Adv. 2020, 10, 2691–2702. [Google Scholar] [CrossRef] [Green Version]
- Durmus, A.; Kasgoz, A.; Ercan, N.; Akın, D.; Sanlı, S. Effect of polyhedral oligomeric silsesquioxane (POSS) reinforced polypropylene (PP) nanocomposite on the microstructure and isothermal crystallization kinetics of polyoxymethylene (POM). Polymer 2012, 53, 5347–5357. [Google Scholar] [CrossRef]
- Zhao, X.; Ye, L. Structure and mechanical properties of polyoxymethylene/multi-walled carbon nanotube composites. Compos. Part B 2011, 42, 926–933. [Google Scholar] [CrossRef]
- Kuciel, S.; Bazan, P.; Liber-Kneć, A.; Gądek-Moszczak, A. Physico-mechanical properties of the poly(oxymethylene) composites reinforced with glass fibers under dynamical loading. Polymers 2019, 11, 2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, I.; Shekhar, C.; Alharbi, W.; Abu Khanjer, M.; Khan, R.A.; Alsalme, A. A highly efficient Ag nanoparticle-immobilized alginate-g-polyacrylonitrile hybrid photocatalyst for the degradation of nitrophenols. Polymers 2020, 12, 3049. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Fan, X.; Xu, W.; Zhang, R.; Wu, G. Biosynthesis of bimetallic Au-Ag nanoparticles using Escherichia coliand its biomedical applications. ACS Biomater. Sci. Eng. 2020, 6, 680–689. [Google Scholar] [CrossRef]
- Satulu, V.; Mitu, B.; Ion, V.; Marascu, V.; Matei, E.; Stancu, C.; Dinescu, G. Combining fluorinated polymers with Ag nanoparticles as a route to enhance optical properties of composite materials. Polymers 2020, 12, 1640. [Google Scholar] [CrossRef]
- Wang, S.; Jia, F.; Wang, X.; Hu, L.; Sun, Y.; Yin, G.; Zhou, T.; Feng, Z.; Kumar, P.; Liu, B. Fabrication of ZnO nanoparticles modified by uniformly dispersed Ag nanoparticles: Enhancement of gas sensing performance. ACS Omega 2020, 5, 5209–5218. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Negi, A.; He, J.; Hu, G.; Tian, S.; Liu, J. Synergistic effects of boron nitride (BN) nanosheets and silver (Ag) nanoparticles on thermal conductivity and electrical properties of epoxy nanocomposites. Polymers 2020, 12, 426. [Google Scholar] [CrossRef] [Green Version]
- Bao, S.Y.; Wang, Z.; Zhang, J.L.; Tian, B.Z. Facet-heterojunction-based z-scheme BiVO4{010} microplates decorated with Ag Br-Ag nanoparticles for the photocatalytic inactivation of bacteria and the decomposition of organic contaminants. ACS Appl. Nano Mater. 2020, 3, 8604–8617. [Google Scholar] [CrossRef]
- Zapata, P.A.; Tamayo, L.; Páez, M.; Cerda, E.; Azócar, I.; Rabagliati, F.M. Nanocomposites based on polyethylene and nanosilver particles produced “by metallocenic “in situ” polymerization: Synthesis, characterization, and antimicrobial behavior. Eur. Polym. J. 2011, 47, 1541–1549. [Google Scholar] [CrossRef]
- Rozilah, A.; Jaafar, C.N.A.; Sapuan, S.M.; Zainol, I.; Ilyas, R.A. The effects of silver nanoparticles compositions on the mechanical, physiochemical, antibacterial, and morphology properties of sugar palm starch bio composites for antibacterial coating. Polymers 2020, 12, 2605. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Chang, Y.W.; Jang, K.S. Morphology, mechanical properties and shape memory effects of polyamide12/polyolefin elastomer blends compatibilized by glycidylisobutyl POSS. Materials 2021, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Luo, H.C.; Moloney, M.; Qiu, J. Adjustable graphene/polyolefin elastomer epsilon-near-zero metamaterials at radiofrequency range. ACS Appl. Mater. Inter. 2020, 12, 22019–22028. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.D.; Han, B.; Du, J.X.; Zhang, J.W.; Wang, J. Effect of multi-monomer grafted polyolefin elastomer/polypropylene on the structure and properties of polypropylene/montmorillonite nanocomposites. J. Macromol. Sci. Phys. 2020, 59, 836–852. [Google Scholar] [CrossRef]
- Tao, G.L.; Liao, X.J.; Fang, J.B.; Xia, Y.P.; Shen, Q.; Shu, Z.W. Synergistic toughening effect of different elastomers on polypropylene. Polym. Mater. Sci. Eng. 2013, 29, 55–59. [Google Scholar]
- Zeng, Y.C.; Liu, Y.; Wang, L.M.; Huang, H.L.; Zhang, X.; Liu, Y.L.; Min, M.H.; Li, Y. Effect of silver nanoparticles on the microstructure, non-isothermal crystallization behavior and antibacterial activity of polyoxymethylene. Polymers 2020, 12, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Y.C.; Liu, Y.; Zhang, X.; Wang, L.M.; Huang, H.L.; Liu, Y.L.; Qi, G.R.; Min, M.H.; Li, Y. Effect of silver nanoparticles on the melting behavior, isothermal crystallization kinetics and morphology of polyoxymethylene. Crystals 2020, 10, 594. [Google Scholar] [CrossRef]
- Wang, B.B.; Yang, Y.; Guo, W.N. Effect of EVOH on the morphology, mechanical and barrier properties of PA6/POE-g-MAH/EVOH ternary blends. Mater. Des. 2012, 40, 185–189. [Google Scholar] [CrossRef]
- Ahn, B.W.; Chi, Y.S.; Kang, T.J. Preparation and characterization of multi-walled carbon nanotube/poly(ethylene terephthalate) nanoweb. J. Appl. Polym. Sci. 2008, 110, 4055. [Google Scholar] [CrossRef]
- Chae, D.W.; Shim, K.B.; Kim, B.C. Effects of silver nanoparticles on the dynamic crystallization and physical properties of syndiotactic polypropylene. J. Appl. Polym. Sci. 2008, 109, 2942–2947. [Google Scholar] [CrossRef]
- Mbhele, Z.H.; Salemane, M.G.; Van Sittert, C.G.C.E.; Nedeljković, J.M.; Djoković, V.; Luyt, A.S. Fabrication and characterization of silver-polyvinyl alcohol nanocomposites. Chem. Mater. 2003, 15, 5019–5024. [Google Scholar] [CrossRef]
- Kumar, G.; Neelakantan, N.R.; Subramanian, N. Polyacetal and thermoplastic polyurethane elastomer toughened polyacetal: Crystallinity and fracture mechanics. J. Mater. Sci. 1995, 30, 1480–1486. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.P.; Wu, Q.; Mao, Z.P. The influence of synergistic effects of hexakis(4-nitrophenoxy) cyclotriphosphazene and POE-g-MA on anti-dripping and flame retardancy of PET. J. Ind. Eng. Chem. 2013, 3, 993–999. [Google Scholar] [CrossRef]
- Zhu, B.D.; Li, W.J.; Song, J.H.; Wang, J. Structure and properties of polypropylene/polyolefin elastomer/organic montmorillonite nanocomposites. J. Macromol. Sci. Phys. 2019, 58, 73–87. [Google Scholar] [CrossRef]
- Grigalovica, A.; Meri, R.M.; Zicans, J.; Grabis, J.; Aniskevich, A. Aging of nanosized ZnO modified polyoxymethylene blends with ethylene octene copolymer. Polym.-Plast. Technol. 2015, 54, 1201–1206. [Google Scholar] [CrossRef]
- Pagano, C.; Surace, R.; Bellantone, V.; Baldi, F.; Fassi, I. Mechanical characterization and replication quality analysis of micro-injected parts made of carbon nanotube/polyoxymethylene nanocomposites. J. Compos. Mater. 2017, 52, 645–657. [Google Scholar] [CrossRef]
- Papageorgion, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Santis, F.D.; Gnerre, C.; Nobile, M.R.; Lamberti, G. The rheological and crystallization behavior of polyoxymethylene. Polym. Test. 2017, 57, 203–208. [Google Scholar] [CrossRef]








| Sample Code (Abbreviation) | POM (wt.%) | Ag (wt.%) | MAH-g-POE (wt.%) |
|---|---|---|---|
| POM | 100 | 0 | 0 |
| 1P-A | 99.9 | 0.1 | 0 |
| 2P-A | 99.5 | 0.5 | 0 |
| 3P-A | 99 | 1 | 0 |
| 4P-A | 98 | 2 | 0 |
| 1P-A-M | 99 | 0.1 | 0.9 |
| 2P-A-M | 95 | 0.5 | 4.5 |
| 3P-A-M | 90 | 1 | 9 |
| 4P-A-M | 85 | 1.5 | 13.5 |
| 5P-A-M | 80 | 2 | 18 |
| Samples | Tm (°C) | Tc (°C) | ΔHm (J/g) a | XC (%) b |
|---|---|---|---|---|
| POM | 168.24 | 140.64 | 152.94 | 46.91 |
| 1P-A-M | 168.83 | 140.83 | 159.29 | 48.86 |
| 2P-A-M | 167.91 | 141.81 | 143.89 | 44.13 |
| 3P-A-M | 167.23 | 142.13 | 138.64 | 42.52 |
| 4P-A-M | 168.81 | 140.71 | 130.71 | 40.01 |
| 5P-A-M | 169.31 | 140.15 | 124.11 | 38.07 |
| Samples | Tensile Strength (MPa) | Elongation at Break (%) | Young Modulus (MPa) | Notched Impact Strength (kJ/m2) |
|---|---|---|---|---|
| POM | 50.03 | 48.05 | 1293.91 | 5.57 |
| 1P-A | 51.79 | 46.31 | 1338.43 | 5.15 |
| 2P-A | 53.18 | 44.88 | 1374.11 | 4.95 |
| 3P-A | 56.12 | 43.94 | 1450.39 | 4.87 |
| 4P-A | 52.27 | 44.92 | 1350.89 | 4.91 |
| 1P-A-M | 54.78 | 49.78 | 1415.76 | 5.92 |
| 2P-A-M | 48.69 | 58.17 | 1255.36 | 6.71 |
| 3P-A-M | 46.62 | 60.66 | 1204.87 | 7.34 |
| 4P-A-M | 44.92 | 64.02 | 1163.93 | 8.98 |
| 5P-A-M | 43.27 | 58.72 | 1119.29 | 7.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, X.; Gao, Q.; Huang, H.; Liu, Y.; Min, M.; Wang, L. Structure and Properties of Polyoxymethylene/Silver/Maleic Anhydride-Grafted Polyolefin Elastomer Ternary Nanocomposites. Polymers 2021, 13, 1954. https://doi.org/10.3390/polym13121954
Liu Y, Zhang X, Gao Q, Huang H, Liu Y, Min M, Wang L. Structure and Properties of Polyoxymethylene/Silver/Maleic Anhydride-Grafted Polyolefin Elastomer Ternary Nanocomposites. Polymers. 2021; 13(12):1954. https://doi.org/10.3390/polym13121954
Chicago/Turabian StyleLiu, Yang, Xun Zhang, Quanxin Gao, Hongliang Huang, Yongli Liu, Minghua Min, and Lumin Wang. 2021. "Structure and Properties of Polyoxymethylene/Silver/Maleic Anhydride-Grafted Polyolefin Elastomer Ternary Nanocomposites" Polymers 13, no. 12: 1954. https://doi.org/10.3390/polym13121954
APA StyleLiu, Y., Zhang, X., Gao, Q., Huang, H., Liu, Y., Min, M., & Wang, L. (2021). Structure and Properties of Polyoxymethylene/Silver/Maleic Anhydride-Grafted Polyolefin Elastomer Ternary Nanocomposites. Polymers, 13(12), 1954. https://doi.org/10.3390/polym13121954