In Vivo Biological Evaluation of Biodegradable Nanofibrous Membranes Incorporated with Antibiofilm Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrospinning Materials
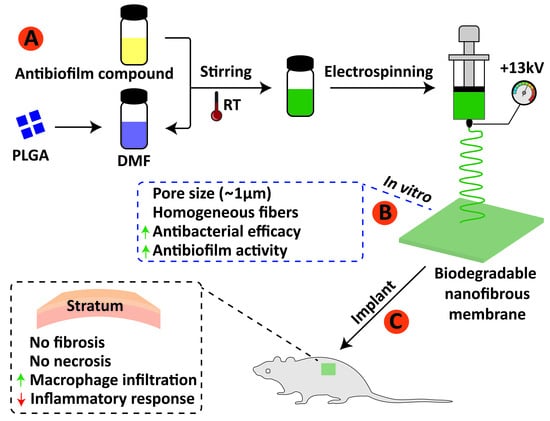
2.2. Electrospinning Process
2.3. Other Biomaterials
2.4. Bacterial Strains and Growth Condition
2.5. Biofilm Formation and Assay
2.6. Surface Analysis of Electrospun Membranes
2.7. Surface Morphology and Fibers Diameter
2.8. Experimental Groups
2.9. Ethical Principles and Conduct in the Care and Use of Animals
2.10. Animals Welfare
2.11. Characterization of Animal
2.12. Anesthesia and Surgical Procedures
2.13. Samples Collection and Materials Processing
2.14. Microscopic Descriptive Analysis
2.15. Evaluation of the Local Biological Effects of Implantation of the Biomaterials. Semiquantitative Histological Analysis: ISO 10993-6:2016/Part 6/Annex E
2.16. Statistical Analysis
3. Results
3.1. Surface Morphology, Diameter Distribution and Pore Size of Electrospun Fibers
3.2. Antibiofilm Analysis
3.3. Microscopic Descriptive Analysis
3.3.1. 1-Week Post-Implantation
3.3.2. 3-Weeks Post-Implantation
3.3.3. 9-Weeks Post-Implantation
3.4. Semiquantitative Histological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dahlin, C.; Linde, A.; Gottlow, J.; Nyman, S. Healing of Bone Defects by Guided Tissue Regeneration. Plast. Reconstr. Surg. 1988, 81, 672–676. [Google Scholar] [CrossRef]
- Buser, D.; Brägger, U.; Lang, N.P.; Nyman, S. Regeneration and enlargement of jaw bone using guided tissue regeneration. Clin. Oral Implant. Res. 1990, 1, 22–32. [Google Scholar] [CrossRef]
- Takata, T.; Miyauchi, M.; Wang, H.-L. Migration of osteoblastic cells on various guided bone regeneration membranes. Clin. Oral Implant. Res. 2001, 12, 332–338. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Schwarz, F. Regeneration of periodontal tissues: Combinations of barrier membranes and grafting materials - biological foundation and preclinical evidence: A systematic review. J. Clin. Periodontol. 2008, 35, 106–116. [Google Scholar] [CrossRef]
- de Santana, R.B.; de Mattos, C.M.L.; Francischone, C.E.; van Dyke, T. Superficial Topography and Porosity of an Absorbable Barrier Membrane Impacts Soft Tissue Response in Guided Bone Regeneration. J. Periodontol. 2010, 81, 926–933. [Google Scholar] [CrossRef]
- Rocchietta, I.; Fontana, F.; Simion, M. Clinical outcomes of vertical bone augmentation to enable dental implant placement: A systematic review. J. Clin. Periodontol. 2008, 35, 203–215. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.-M.G.; Kowolik, M.J.; Janowski, G. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly (lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Wang, H.-L.; Miyauchi, M. Attachment, proliferation and differentiation of periodontal ligament cells on various guided tissue regeneration membranes. J. Periodontal Res. 2001, 36, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Passos, P.C.; Moro, J.; Barcelos, R.C.S.; Da Rosa, H.Z.; Vey, L.T.; Bürguer, M.E.; Maciel, R.M.; Danesi, C.C.; Edwards, P.C.; Bottino, M.C.; et al. Nanofibrous antibiotic-eluting matrices: Biocompatibility studies in a rat model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 306–315. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Janowski, G. A novel spatially designed and functionally graded electrospun membrane for periodontal regeneration. Acta Biomater. 2011, 7, 216–224. [Google Scholar] [CrossRef]
- Unalan, I.; Endlein, S.J.; Slavik, B.; Buettner, A.; Goldmann, W.H.; Detsch, R.; Boccaccini, A.R. Evaluation of Electrospun Poly (ε-Caprolactone)/Gelatin Nanofiber Mats Containing Clove Essential Oil for Antibacterial Wound Dressing. Pharmaceutics 2019, 11, 570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.I.; Hwang, T.I.; Aguilar, L.E.; Park, C.H.; Kim, C.S. A Controlled Design of Aligned and Random Nanofibers for 3D Bi-functionalized Nerve Conduits Fabricated via a Novel Electrospinning Set-up. Sci. Rep. 2016, 6, 23761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.-J.; Cooper, J.A.; Mauck, R.; Tuan, R.S. Fabrication and characterization of six electrospun poly (α-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006, 2, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, A.; Vignudelli, E.; Napolitano, A.; Marchetti, C.; Corinaldesi, G. Evaluation of complication rates and vertical bone gain after guided bone regeneration with non-resorbable membranes versus titanium meshes and resorbable membranes. A randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2017, 19, 821–832. [Google Scholar] [CrossRef] [Green Version]
- Filippo Fontana, D.D.S.; Rocchietta, I.; Massimo Simion, M.D. Complications in guided bone regeneration. In Dental Implant Complications: Etiology, Prevention, and Treatment, 2nd ed.; Wiley online library: Hoboken, NJ, USA, 2015. [Google Scholar]
- Brady, R.A.; Leid, J.G.; Calhoun, J.H.; Costerton, J.W.; Shirtliff, M.E. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol. Med Microbiol. 2008, 52, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Marsh, P.; Lewis, M.; Williams, D.; Martin, M. Oral Microbiology, 5th ed.; Elsevier: Edinburgh, UK, 2009. [Google Scholar]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Seebach, E.; Kubatzky, K.F. Chronic Implant-Related Bone Infections—Can Immune Modulation be a Therapeutic Strategy? Front. Immunol. 2019, 10, 1724. [Google Scholar] [CrossRef] [Green Version]
- Dai, T.; Vrahas, M.S.; Murray, C.K.; Hamblin, M.R. Ultraviolet C irradiation: An alternative antimicrobial approach to localized infections? Expert Rev. Anti-infective Ther. 2012, 10, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Scaffaro, R.; Maio, A.; D’Arrigo, M.; Lopresti, F.; Marino, A.; Bruno, M.; Nostro, A. Flexible mats as promising antimicrobial systems via integration of Thymus capitatus (L.) essential oil into PLA. Futur. Microbiol. 2020, 15, 1379–1392. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Gulino, F.E.; Lopresti, F. Structure–property relationship and controlled drug release from multiphasic electrospun carvacrol-embedded polylactic acid/polyethylene glycol and polylactic acid/polyethylene oxide nanofiber mats. J. Ind. Text. 2020, 49, 943–966. [Google Scholar] [CrossRef]
- Eleung, V.; Edufour, D.; Lévesque, C.M. Death and survival in Streptococcus mutans: Differing outcomes of a quorum-sensing signaling peptide. Front. Microbiol. 2015, 6, 1176. [Google Scholar]
- Xavier, J.; Geremias, T.; Montero, J.; Vahey, B.; Benfatti, C.; Souza, J.; Magini, R.; Pimenta, A. Lactam inhibiting Streptococcus mutans growth on titanium. Mater. Sci. Eng. C 2016, 68, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Montero, J.F.; Tajiri, H.A.; Barra, G.M.D.O.; Fredel, M.C.; Benfatti, C.A.; Magini, R.S.; Pimenta, A.L.; Souza, J.C. Biofilm behavior on sulfonated poly(ether-ether-ketone) (sPEEK). Mater. Sci. Eng. C 2017, 70, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Bassler, B. Quorum Sensing in Bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, Y.-J.; Choi, Y.-J.; Lee, S.-H.; Jun, H.-K.; Choi, B.-K. Autoinducer 2 of Fusobacterium nucleatum as a target molecule to inhibit biofilm formation of periodontopathogens. Arch. Oral Biol. 2013, 58, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Givskov, M. Quorum-sensing blockade as a strategy for enhancing host defences against bacterial pathogens. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Cadavid, E.; Echeverri, F. The Search for Natural Inhibitors of Biofilm Formation and the Activity of the Autoinductor C6-AHL in Klebsiella pneumoniae ATCC 13884. Biomolecules 2019, 9, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayumov, A.; Khakimullina, E.N.; Sharafutdinov, I.; Trizna, E.Y.; Latypova, L.Z.; Lien, H.T.; Margulis, A.B.; Bogachev, M.; Kurbangalieva, A.R. Inhibition of biofilm formation in Bacillus subtilis by new halogenated furanones. J. Antibiot. 2014, 68, 297–301. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Kim, J.; Park, H.Y.; Park, H.J.; Lee, J.H.; Kim, C.K.; Yoon, J. Furanone derivatives as quorum-sensing antagonists of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2008, 80, 37–47. [Google Scholar] [CrossRef]
- Park, J.S.; Ryu, E.-J.; Li, L.; Choi, B.-K.; Kim, B.M. New bicyclic brominated furanones as potent autoinducer-2 quorum-sensing inhibitors against bacterial biofilm formation. Eur. J. Med. Chem. 2017, 137, 76–87. [Google Scholar] [CrossRef]
- He, Z.; Wang, Q.; Hu, Y.; Liang, J.; Jiang, Y.; Ma, R.; Tang, Z.; Huang, Z. Use of the quorum sensing inhibitor furanone C-30 to interfere with biofilm formation by Streptococcus mutans and its luxS mutant strain. Int. J. Antimicrob. Agents 2012, 40, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Bordini, E.A.F.; Tonon, C.C.; Francisconi, R.S.; Magalhães, F.A.C.; Huacho, P.M.M.; Bedran, T.L.; Pratavieira, S.; Spolidorio, L.C.; Spolidorio, D.P. Antimicrobial effects of terpinen-4-ol against oral pathogens and its capacity for the modulation of gene expression. Biofouling 2018, 34, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Comin, V.M.; Lopes, L.Q.; Quatrin, P.M.; de Souza, M.E.; Bonez, P.C.; Pintos, F.G.; Raffin, R.; Vaucher, R.D.A.; Martinez, D.S.T.; Santos, R.C.V. Influence of Melaleuca alternifolia oil nanoparticles on aspects of Pseudomonas aeruginosa biofilm. Microb. Pathog. 2016, 93, 120–125. [Google Scholar] [CrossRef]
- de Souza, M.E.; Clerici, D.J.; Verdi, C.M.; Fleck, G.; Quatrin, P.M.; Spat, L.E.; Bonez, P.C.; Dos Santos, C.F.; Antoniazzi, R.P.; Zanatta, F.B. Antimicrobial activity of Melaleuca alternifolia nanoparticles in polymicrobial biofilm in situ. Microb. Pathog. 2017, 113, 432–437. [Google Scholar] [CrossRef]
- Kolenbrander, P.E. Oral Microbial Communities: Biofilms, Interactions, and Genetic Systems. Annu. Rev. Microbiol. 2000, 54, 413–437. [Google Scholar] [CrossRef]
- Geremias, T.C.; Batistella, M.A.; Magini, R.R.S.; de Souza, S.M.A.G.U.; Franco, C.V.; Barbosa, L.C.A.; Pereira, U.A.; Hinestroza, J.P.; Pimenta, A.L.; de Souza, A.A.U. Functionalization of poly (lactic-co-glycolic acid) nanofibrous membranes with antibiofilm compounds. Can. J. Chem. Eng. 2021, 1–11. [Google Scholar]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. J. Pharmacol. Pharmacother. 2010, 1, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.J.; Clutton, R.E.; Lilley, E.; Hansen, K.E.A.; Brattelid, T. PREPARE: Guidelines for planning animal research and testing. Lab. Anim. 2017, 52, 135–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sealed Envelope. Available online: https://www.stat.ubc.ca/~rollin/stats/ssize/n2.html (accessed on 15 May 2018).
- Banerjee, S.; Bagchi, B.; Pal, K.; Bhandary, S.; Kool, A.; Hoque, N.A.; Biswas, P.; Thakur, P.; Das, K.; Karmakar, P.; et al. Essential oil impregnated luminescent hydroxyapatite: Antibacterial and cytotoxicity studies. Mater. Sci. Eng. C 2020, 116, 111190. [Google Scholar] [CrossRef]
- Cheng, Y.; Mei, S.; Kong, X.; Liu, X.; Gao, B.; Chen, B.; Wu, J. Long-term antibacterial activity of a composite coating on titanium for dental implant application. J. Biomater. Appl. 2021, 35, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, E.; Karakeçili, A.; Demirtaş, T.T.; Gümüşderelioğlu, M. Preparation of bioactive and antimicrobial PLGA membranes by magainin II/EGF functionalization. Int. J. Biol. Macromol. 2016, 86, 162–168. [Google Scholar] [CrossRef]
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Mori, C.L.S.D.O.; Dos Passos, N.A.; Oliveira, J.; Altoé, T.F.; Mori, F.A.; Mattoso, L.H.C.; Scolforo, J.R.; Tonoli, G. Nanostructured Polylactic Acid/Candeia Essential Oil Mats Obtained by Electrospinning. J. Nanomater. 2015, 2015, 33. [Google Scholar] [CrossRef]
- Unalan, I.; Slavik, B.; Buettner, A.; Goldmann, W.H.; Frank, G.; Boccaccini, A.R. Physical and Antibacterial Properties of Peppermint Essential Oil Loaded Poly (ε-caprolactone) (PCL) Electrospun Fiber Mats for Wound Healing. Front. Bioeng. Biotechnol. 2019, 7, 346. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.H.; Kim, J.H.; Song, K.S.; Jeon, B.H.; Yoon, J.H.; Seo, T.B.; Namgung, U.; Lee, I.W.; Lee, J.H. Peripheral nerve regeneration within an asymmetrically porous PLGA/Pluronic F127 nerve guide conduit. Biomaterials 2008, 29, 1601–1609. [Google Scholar] [CrossRef]
- Behring, J.; Junker, R.; Walboomers, X.F.; Chessnut, B.; Jansen, J.A. Toward guided tissue and bone regeneration: Morphology, attachment, proliferation, and migration of cells cultured on collagen barrier membranes. A systematic review. Odontology 2008, 96, 1–11. [Google Scholar] [CrossRef]
- Park, J.K.; Yeom, J.; Oh, E.J.; Reddy, M.; Kim, J.Y.; Cho, D.-W.; Lim, H.P.; Kim, N.S.; Park, S.W.; Shin, H.-I.; et al. Guided bone regeneration by poly (lactic-co-glycolic acid) grafted hyaluronic acid bi-layer films for periodontal barrier applications. Acta Biomater. 2009, 5, 3394–3403. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Thomas, V.; Jose, M.V.; Dean, D.R.; Janowski, G.M. Acellular dermal matrix graft: Synergistic effect of rehydration and natural crosslinking on mechanical properties. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95, 276–282. [Google Scholar] [CrossRef]
- Ratiu, C.; Brocks, M.; Costea, T.; Moldovan, L.; Cavalu, S. PRGF-Modified Collagen Membranes for Guided Bone Regeneration: Spectroscopic, Microscopic and Nano-Mechanical Investigations. Appl. Sci. 2019, 9, 1035. [Google Scholar] [CrossRef] [Green Version]
- Cavalu, S.; Roiu, G.; Pop, O.; Heredea, D.; Costea, T.; Costea, C. Nano-Scale Modifications of Amniotic Membrane Induced by UV and Antibiotic Treatment: Histological, AFM and FTIR Spectroscopy Evidence. Materials 2021, 14, 863. [Google Scholar] [CrossRef]
- Kikuchi, M.; Koyama, Y.; Yamada, T.; Imamura, Y.; Okada, T.; Shirahama, N.; Akita, K.; Takakuda, K.; Tanaka, J. Development of guided bone regeneration membrane composed of β-tricalcium phosphate and poly (l-lactide-co-glycolide-co-ε-caprolactone) composites. Biomaterials 2004, 25, 5979–5986. [Google Scholar] [CrossRef]
- Tessmar, J.K.V.; Holland, T.A.; Mikos, A.G. Salt Leaching for Polymer Scaffolds: Laboratory-Scale Manufacture of Cell Carriers. In Scaffolding in Tissue Engineering; Ma, P., Elisseeff, J., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 111–124. [Google Scholar]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Z.; Triffitt, J.T. A review on macrophage responses to biomaterials. Biomed. Mater. 2006, 1, R1. [Google Scholar] [CrossRef]
- Mitragotri, S.; Lahann, J. Physical approaches to biomaterial design. Nat. Mater. 2009, 8, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoornaert, A.; D’Arros, C.; Heymann, M.-F.; Layrolle, P. Biocompatibility, resorption and biofunctionality of a new synthetic biodegradable membrane for guided bone regeneration. Biomed. Mater. 2016, 11, 045012. [Google Scholar] [CrossRef] [PubMed]
- Rich, A.; Harris, A. Anomalous preferences of cultured macrophages for hydrophobic and roughened substrata. J. Cell Sci. 1981, 50, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rothamel, D.; Schwarz, F.; Sculean, A.; Herten, M.; Scherbaum, W.; Becker, J. Biocompatibility of various collagen membranes in cultures of human PDL fibroblasts and human osteoblast-like cells. Clin. Oral Implant. Res. 2004, 15, 443–449. [Google Scholar] [CrossRef]
- Miron, R.J.; Bosshardt, D.D. OsteoMacs: Key players around bone biomaterials. Biomaterials 2016, 82, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Feng, Y.; Cheng, H.; Li, D. The role of macrophages in osseointegration of dental implants: An experimental study in vivo. J. Biomed. Mater. Res. Part A 2020, 108, 2206–2216. [Google Scholar] [CrossRef]
- Miron, R.J.; Zohdi, H.; Fujioka-Kobayashi, M.; Bosshardt, D.D. Giant cells around bone biomaterials: Osteoclasts or multi-nucleated giant cells? Acta Biomater. 2016, 46, 15–28. [Google Scholar] [CrossRef]
- Böstman, O.M.; Pihlajamäki, H. Adverse tissue reactions to bioabsorbable fixation devices. Clin. Orthop. Relat. Res. 2000, 216–227. [Google Scholar] [CrossRef] [Green Version]
- Ceri, H.; Olson, M.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New Technology for Rapid Determination of Antibiotic Susceptibilities of Bacterial Biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharafutdinov, I.S.; Pavlova, A.S.; Akhatova, F.S.; Khabibrakhmanova, A.M.; Rozhina, E.V.; Romanova, Y.J.; Fakhrullin, R.; Lodochnikova, O.A.; Kurbangalieva, A.R.; Bogachev, M.I.; et al. Unraveling the Molecular Mechanism of Selective Antimicrobial Activity of 2 (5H)-Furanone Derivative against Staphylococcus aureus. Int. J. Mol. Sci. 2019, 20, 694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proctor, C.R.; McCarron, P.A.; Ternan, N.G. Furanone quorum-sensing inhibitors with potential as novel therapeutics against Pseudomonas aeruginosa. J. Med Microbiol. 2020, 69, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Bernabè, G.; Filippini, R.; Piovan, A. In Vitro Antimicrobial Activities of Commercially Available Tea Tree (Melaleuca alternifolia) Essential Oils. Curr. Microbiol. 2019, 76, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-M.; Zhou, H.-Y.; Wu, Y.; Wang, J.; Liu, Q.; Mei, Y.-F. In Vitro Evaluation of the Antibacterial Properties of Tea Tree Oil on Planktonic and Biofilm-Forming Streptococcus mutans. AAPS PharmSciTech 2020, 21, 227. [Google Scholar] [CrossRef] [PubMed]
- Casarin, M.; Pazinatto, J.; Santos, R.C.V.; Zanatta, F.B. Melaleuca alternifolia and its application against dental plaque and periodontal diseases: A systematic review. Phytother. Res. 2018, 32, 230–242. [Google Scholar] [CrossRef]
- Bai, M.Y.; Chou, T.C.; Tsai, J.C.; Yu, W.C. The effect of active ingredient-containing chitosan/polycaprolactone nonwoven mat on wound healing: In vitro and in vivo studies. J. Biomed. Mater. Res. Part A 2014, 102, 2324–2333. [Google Scholar] [CrossRef]
- Baveja, J.; Willcox, M.; Hume, E.; Kumar, N.; Odell, R.; Poole-Warren, L. Furanones as potential anti-bacterial coatings on biomaterials. Biomaterials 2004, 25, 5003–5012. [Google Scholar] [CrossRef] [PubMed]
- Baveja, J.; Li, G.; Nordon, R.; Hume, E.; Kumar, N.; Willcox, M.; Poole-Warren, L. Biological performance of a novel synthetic furanone-based antimicrobial. Biomaterials 2004, 25, 5013–5021. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geremias, T.C.; Sartoretto, S.C.; Batistella, M.A.; Souza, A.A.U.d.; Alves, A.T.N.N.; Uzeda, M.J.P.; Calasans-Maia, J.; Montemezzi, P.; Mourão, C.F.d.A.B.; Calasans-Maia, M. In Vivo Biological Evaluation of Biodegradable Nanofibrous Membranes Incorporated with Antibiofilm Compounds. Polymers 2021, 13, 2457. https://doi.org/10.3390/polym13152457
Geremias TC, Sartoretto SC, Batistella MA, Souza AAUd, Alves ATNN, Uzeda MJP, Calasans-Maia J, Montemezzi P, Mourão CFdAB, Calasans-Maia M. In Vivo Biological Evaluation of Biodegradable Nanofibrous Membranes Incorporated with Antibiofilm Compounds. Polymers. 2021; 13(15):2457. https://doi.org/10.3390/polym13152457
Chicago/Turabian StyleGeremias, Thaise C., Suelen C. Sartoretto, Marcos A. Batistella, Antônio A. Ulson de Souza, Adriana T. N. N. Alves, Marcelo J.P. Uzeda, Jose Calasans-Maia, Pietro Montemezzi, Carlos Fernando de Almeida Barros Mourão, and Monica Calasans-Maia. 2021. "In Vivo Biological Evaluation of Biodegradable Nanofibrous Membranes Incorporated with Antibiofilm Compounds" Polymers 13, no. 15: 2457. https://doi.org/10.3390/polym13152457
APA StyleGeremias, T. C., Sartoretto, S. C., Batistella, M. A., Souza, A. A. U. d., Alves, A. T. N. N., Uzeda, M. J. P., Calasans-Maia, J., Montemezzi, P., Mourão, C. F. d. A. B., & Calasans-Maia, M. (2021). In Vivo Biological Evaluation of Biodegradable Nanofibrous Membranes Incorporated with Antibiofilm Compounds. Polymers, 13(15), 2457. https://doi.org/10.3390/polym13152457