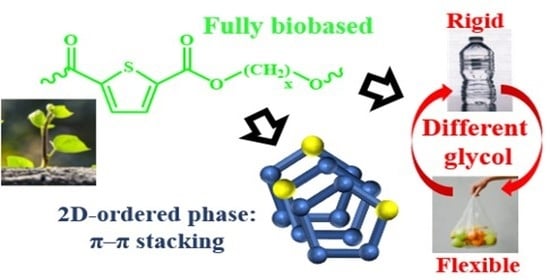
Poly(Alkylene 2,5-Thiophenedicarboxylate) Polyesters: A New Class of Bio-Based High-Performance Polymers for Sustainable Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Molecular Characterisation
2.4. Film by Compression Moulding
2.5. Thermal and Structural Characterisation
2.6. Mechanical Characterisation
2.7. Gas Barrier Properties Evaluation
3. Results and Discussion
3.1. Synthesis and Molecular Characterisation
3.2. Thermal Characterisation
3.2.1. Powder Samples
3.2.2. Compression-Moulded Films
3.3. Mechanical Characterisation
3.4. Gas Barrier Properties Evaluation
4. Conclusions
- Mesophase formation is favoured in the case of macromolecular chains, which are mobile at room temperature but have reduced crystallising capability. If the glycol subunit is long enough, 3D crystalline phase formation appears prevalent
- Mesophases and crystalline phases compete with each other, i.e., each one develops at the expense of the other
- Type, number, and amount of ordered phases have a huge impact on the final functional properties (mechanical and gas barrier)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plastics Europe-Plastics the Facts. 2020. Available online: https://www.plasticseurope.org (accessed on 12 June 2021).
- European Bioplastics-Bioplastics Market Data. 2020. Available online: https://docs.european-bioplastics.org (accessed on 12 June 2021).
- European Commission-A European Strategy for Plastics in a Circular Economy. 2015. Available online: https://ec.europa.eu/environment/circular-economy/pdf/plastics-strategy-brochure.pdf (accessed on 12 June 2021).
- Top Value Added Chemicals from Biomass Volume I-Results of Screening for Potential Candidates from Sugars and Synthesis Gas. 2004. Available online: https://www.nrel.gov/docs/fy04osti/35523.pdf (accessed on 12 June 2021).
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Chain Mobility, Thermal, and Mechanical Properties of Poly(ethylene furanoate) Compared to Poly(ethylene terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Burgess, S.K.; Mikkilineni, D.S.; Yu, D.B.; Kim, D.J.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Water sorption in poly(ethylene furanoate) compared to poly(ethylene terephthalate). Part 1: Equilibrium sorption. Polymer 2014, 55, 6861–6869. [Google Scholar] [CrossRef]
- Burgess, S.K.; Mikkilineni, D.S.; Yu, D.B.; Kim, D.J.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Water sorption in poly(ethylene furanoate) compared to poly(ethylene terephthalate). Part 2: Kinetic sorption. Polymer 2014, 55, 6870–6882. [Google Scholar] [CrossRef]
- Burgess, S.K.; Kriegel, R.M.; Koros, W.J. Carbon Dioxide Sorption and Transport in Amorphous Poly(ethylene furanoate). Macromolecules 2015, 48, 2184–2193. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Jia, Z.; Liu, Y.; Sun, L.; Zhu, J. Synthesis of bio-based poly(ethylene 2,5-furandicarboxylate) copolyesters: Higher glass transition temperature, better transparency, and good barrier properties. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3298–3307. [Google Scholar] [CrossRef]
- Van Putten, R.J.; Van der Waal, J.C.; De Jong, E.; Rasrendra, C.B.; Heeres, H.J.; De Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Zhi, W.; Hu, Y.; Liang, M.; Liu, Y.; Li, J.; Yin, J.; Shi, Y. Solid-liquid equilibrium and thermodynamic of 2,5-thiophenedicarboxylic acid in different organic solvents. Fluid Phase Equilib. 2014, 375, 110–114. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Q.; Cao, C.; Cheng, L.; Shi, Y.; Yang, W.; Hu, Y. Solubility and solution thermodynamics of 2,5-thiophenedicarboxylic acid in (water + ethanol) binary solvent mixtures. Thermochim. Acta 2014, 592, 52–57. [Google Scholar] [CrossRef]
- Polen, T.; Spelberg, M.; Bott, M. Toward biotechnological production of adipic acid and precursors from biorenewables. J. Biotechnol. 2013, 167, 75–84. [Google Scholar] [CrossRef]
- Corona, A.; Biddy, M.J.; Vardon, D.R.; Birkved, M.; Hauschild, M.Z.; Beckham, G.T. Life cycle assessment of adipic acid production from lignin. Green Chem. 2018, 20, 3857–3866. [Google Scholar] [CrossRef] [Green Version]
- Guidotti, G.; Soccio, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Munari, A. Poly(propylene 2,5-thiophenedicarboxylate) vs. poly(propylene 2,5-furandicarboxylate): Two examples of high gas barrier bio-based polyesters. Polymers 2018, 10, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidotti, G.; Gigli, M.; Soccio, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Munari, A. Poly(butylene 2,5-thiophenedicarboxylate): An added value to the class of high gas barrier biopolyesters. Polymers 2018, 10, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidotti, G.; Gigli, M.; Soccio, M.; Lotti, N.; Salatelli, E.; Gazzano, M.; Siracusa, V.; Munari, A. Tailoring poly(butylene 2,5- thiophenedicarboxylate) features by the introduction of adipic acid co-units: Biobased and biodegradable aliphatic/aromatic polyesters. Polymer 2018, 145, 11–20. [Google Scholar] [CrossRef]
- Guidotti, G.; Gigli, M.; Soccio, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Munari, A. Ordered structures of poly(butylene 2,5-thiophenedicarboxylate) and their impact on material functional properties. Eur. Polym. J. 2018, 106, 284–290. [Google Scholar] [CrossRef]
- Wang, G.Q.; Liang, Y.; Jiang, M.; Zhang, Q.; Wang, R.; Wang, H.H.; Zhou, G.Y. Synthesis and characterization of bio-based polyesters from 2,5-thiophenedicarboxylic acid. Polym. Degrad. Stab. 2019, 168, 108942. [Google Scholar] [CrossRef]
- Wang, G.Q.; Jiang, M.; Zhang, Q.; Wang, R.; Liang, Q.D.; Wang, H.H.; Zhou, G.Y. Partially bio-based and tough polyesters, poly(ethylene 2,5-thiophenedicarboxylate-co-1,4-cyclohexanedimethylene 2,5-thiophenedicarboxylate)s. Express Polym. Lett. 2019, 13, 938–947. [Google Scholar] [CrossRef]
- Wang, G.Q.; Liang, Y.; Jiang, M.; Zhang, Q.; Wang, R.; Wang, H.H.; Zhou, G.Y. High Tg and tough poly(butylene 2,5- thiophenedicarboxylate-co-1,4-cyclohexanedimethylene 2,5-thiophenedicarboxylate)s: Synthesis and characterization. J. Appl. Polym. Sci. 2020, 137, 48634. [Google Scholar] [CrossRef]
- Wang, G.Q.; Jiang, M.; Zhang, Q.; Wang, R.; Liang, Q.D.; Zhou, G.Y. New bio-based copolyesters poly(trimethylene 2,5-thiophenedicarboxylate-co-trimethylene terephthalate): Synthesis, crystallization behavior, thermal and mechanical properties. Polymer 2019, 173, 27–33. [Google Scholar] [CrossRef]
- Wang, G.Q.; Jiang, M.; Zhang, Q.; Wang, R.; Liang, Q.D.; Zhou, G.Y. New bio-based copolyesters derived from 1,4-butanediol, terephthalic acid and 2,5-thiophenedicarboxylic acid: Synthesis, crystallization behavior, thermal and mechanical properties. Polym. Test. 2019, 75, 213–219. [Google Scholar] [CrossRef]
- Wang, J.G.; Zhang, X.Q.; Shen, A.; Zhu, J.; Song, P.A.; Wang, H.; Liu, X.Q. Synthesis and Properties Investigation of Thiophene-aromatic Polyesters: Potential Alternatives for the 2,5-Furandicarboxylic Acid-based Ones. Chin. J. Polym. Sci. 2020, 38, 1082–1091. [Google Scholar] [CrossRef]
- Ferrario, V.; Pellis, A.; Cespugli, M.; Guebitz, G.M.; Gardossi, L. Nature Inspired Solutions for Polymers: Will Cutinase Enzymes Make Polyesters and Polyamides Greener? Catalysts 2017, 6, 205. [Google Scholar] [CrossRef] [Green Version]
- Pellis, A.; Gamerith, C.; Ghazaryan, G.; Aortner, A.; Herrero Acero, E.; Guebitz, G.M. Ultrasound-enhanced enzymatic hydrolysis of poly(ethylene terephthalate). Bioresour. Technol. 2016, 218, 1298–1302. [Google Scholar] [CrossRef]
- Weinberger, S.; Canadell, J.; Quartinello, F.; Yeniad, B.; Arias, A.; Pellis, A.; Guebitz, G.M. Enzymatic Degradation of Poly(ethylene 2,5-furanoate) Powders and Amorphous Films. Catalysts 2017, 7, 318. [Google Scholar] [CrossRef] [Green Version]
- Gigli, M.; Quartinello, F.; Soccio, M.; Pellis, A.; Lotti, N.; Guebitz, G.M.; Licoccia, S.; Munari, A. Enzymatic hydrolysis of poly(1,4-butylene 2,5-thiophenedicarboxylate) (PBTF) and poly(1,4-butylene 2,5-furandicarboxylate) (PBF) films: A comparison of mechanisms. Environ. Int. 2019, 130, 104852. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, G.; Soccio, M.; García-Gutiérrez, M.C.; Ezquerra, T.; Siracusa, V.; Gutiérrez-Fernández, E.; Munari, A.; Lotti, N. Fully Biobased Superpolymers of 2,5-Furandicarboxylic Acid with Different Functional Properties: From Rigid to Flexible, High Performant Packaging Materials. ACS Sustain. Chem. Eng. 2020, 8, 9558–9568. [Google Scholar] [CrossRef] [PubMed]
- Dobbertin, J.; Hensel, A.; Schick, C. Dielectric spectroscopy and calorimetry in the glass transition region of semi-crystalline poly(ethylene terephthalate). J. Therm. Anal. 1996, 47, 1027–1040. [Google Scholar] [CrossRef]
- Sanz, A.; Nogales, A.; Ezquerra, T.A.; Lotti, N.; Munari, A.; Funari, S.S. Order and segmental mobility during polymer crystallization: Poly(butylene isophthalate). Polymer 2006, 47, 1281–1290. [Google Scholar] [CrossRef] [Green Version]
- Soccio, M.; Lotti, N.; Finelli, L.; Munari, A. Thermal characterization of novel aliphatic polyesters with ether and thioether linkages. e-Polymers 2009, 10, 35. [Google Scholar]
- Guidotti, G.; Soccio, M.; García-Gutiérrez, M.C.; Gutiérrez-Fernández, E.; Ezquerra, T.A.; Siracusa, V.; Munari, A.; Lotti, N. Evidence of a 2D-Ordered Structure in Biobased Poly(pentamethylene furanoate) Responsible for Its Outstanding Barrier and Mechanical Properties. ACS Sustain. Chem. Eng. 2019, 7, 17863–17871. [Google Scholar] [CrossRef]
- Martínez-Tong, D.E.; Soccio, M.; Robles-Hernández, B.; Guidotti, G.; Gazzano, M.; Lotti, N.; Alegria, A. Evidence of Nanostructure Development from the Molecular Dynamics of Poly(pentamethylene 2,5-furanoate). Macromolecules 2020, 53, 10526–10537. [Google Scholar] [CrossRef]
- Rudyak, V.Y.; Gavrilov, A.A.; Guseva, D.V.; Tung, S.H.; Komarov, P.V. Accounting for π–π stacking interactions in the mesoscopic models of conjugated polymers. Mol. Syst. Des. Eng. 2020, 5, 1137. [Google Scholar] [CrossRef]
- Sago, T.; Itagaki, H.; Asano, T. Onset of Forming Ordering in Uniaxially Stretched Poly(ethylene terephthalate) Films Due to π−π Interaction Clarified by the Fluorescence. Macromolecules 2014, 47, 217–226. [Google Scholar] [CrossRef]
- Shanavas, A.; Sathiyaraj, S.; Chandramohan, A.; Narasimhaswamy, T.; Sultan Nasar, A. Isophthalic acid based mesogenic dimers: Synthesis and structural effects on mesophase properties. J. Mol. Struct. 2013, 1038, 126–133. [Google Scholar] [CrossRef]
- Mahendrasingam, A.; Blundell, D.J.; Martin, C.; Urban, V.; Narayanan, T.; Fuller, W. Time resolved WAXS study of the role of mesophase in oriented crystallisation of poly(ethylene terephthalate-co-isophthalate) copolymers. Polymer 2005, 46, 6044–6049. [Google Scholar] [CrossRef]
- Hedenqvist, M.S. Barrier Packaging Materials. In Handbook of Environmental Degradation of Materials, 2nd ed.; Kutz, M., Ed.; Elsevier, Inc.: Amsterdam, The Netherlands, 2012; pp. 840–842. [Google Scholar]
- Quattrosoldi, S.; Lotti, N.; Soccio, M.; Schick, C.; Androsch, R. Stability of Crystal Nuclei of Poly (butylene isophthalate) Formed Near the Glass Transition Temperature. Polymers 2020, 12, 1099. [Google Scholar] [CrossRef] [PubMed]
- Quattrosoldi, S.; Androsch, R.; Janke, A.; Soccio, M.; Lotti, N. Enthalpy Relaxation, Crystal Nucleation and Crystal Growth of Biobased Poly(butylene Isophthalate). Polymers 2020, 12, 235. [Google Scholar] [CrossRef] [Green Version]
- Genovese, L.; Gigli, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Munari, A.; Dalla Rosa, M. Biodegradable Long Chain Aliphatic Polyesters Containing Ether-Linkages: Synthesis, Solid-State, and Barrier Properties. Ind. Eng. Chem. Res. 2014, 53, 10965–10973. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Siracusa, V.; Gazzano, M.; Salatelli, E.; Munari, A.; Lotti, N. Novel Random PBS-Based Copolymers Containing Aliphatic Side Chains for Sustainable Flexible Food Packaging. Polymers 2017, 9, 724. [Google Scholar] [CrossRef] [Green Version]
- Robertson, G.L. Optical, Mechanical and Barrier Properties of Thermoplastics Polymers. In Food Packaging-Principles and Practice, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 91–130. [Google Scholar]
- Mensitieri, G.; Di Maio, E.; Buonocore, G.G.; Nedi, I.; Oliviero, M.; Sansone, L.; Iannace, S. Processing and shelf life issues of selected food packaging materials and structures from renewable resources. Trends Food Sci. Technol. 2011, 22, 72–80. [Google Scholar] [CrossRef]









| Peak Position (ppm) | Mn (Da) | D | ||
|---|---|---|---|---|
| Diacid Subunit | Glycolic Subunit | |||
| PPTF | 7.83 (2H, s) | 2.34 (2H, m) 4.60 (4H, t) | 26,300 | 2.3 |
| PBTF | 7.83 (2H, s) | 1.99 (4H, m) 4.48 (4H, t) | 39,600 | 2.0 |
| PPeTF | 7.70 (2H, s) | 1.58 (2H, m) 1.82 (4H, m) 4.34 (4H, t) | 25,800 | 2.5 |
| PHTF | 7.70 (2H, s) | 1.51 (4H, m) 1.81 (4H, m) 4.32 (4H, t) | 32,400 | 2.3 |
| Tonset °C | Tmax °C | I Scan | II Scan | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tg °C | ∆cp J/g°C | Tcc °C | ∆Hcc J/g | Tm °C | ∆Hm J/g | Tg °C | ∆cp J/g°C | Tcc °C | ∆Hcc J/g | Tm °C | ∆Hm J/g | |||
| PPTF | 375 | 402 | 36 | 0.175 | - | - | 183 | 44 | 36 | 0.323 | 120 | 36 | 183 | 37 |
| PBTF | 391 | 410 | 24 | 0.272 | 87 | 28 | 148 | 28 | 24 | 0.291 | 88 | 27 | 148 | 27 |
| PPeTF | 382 | 402 | 8 | 0.144 | - | - | 52/63 | 19/6 | 8 | 0.308 | - | - | - | - |
| PHTF | 384 | 402 | 7 | 0.008 | - | - | 49/95 | 10/23 | 2 | 0.289 | - | - | - | - |
| E (MPa) | σB (MPa) | εB (%) | O2-TR (cm3 cm m−2 d−1 atm−1) | CO2-TR (cm3 cm m−2 d−1 atm−1) | |
|---|---|---|---|---|---|
| PPTF | 1550 ± 125 | 13± 3 | 1.2 ± 0.3 | 0.021 ± 0.003 | 0.024 ± 0.002 |
| PBTF | 47 ± 3 | 19 ± 2 | 580 ± 60 | 0.003 ± 0.001 | 0.017 ± 0.001 |
| PPeTF | 1.9 ± 0.3 * 391 ± 26 | 0.5 ± 0.1 * 14 ± 2 | 1650 ± 110 * 17 ± 3 | 0.288 ± 0.035 | 0.753 ± 0.125 |
| PHTF | 3.5 ± 0.5 * 299 ± 22 | 0.5 ± 0.1 * 15 ± 1 | 674 ± 9 * 32 ± 3 | 0.404 ± 0.101 | 1.62 ± 0.110 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guidotti, G.; Soccio, M.; Gazzano, M.; Siracusa, V.; Lotti, N. Poly(Alkylene 2,5-Thiophenedicarboxylate) Polyesters: A New Class of Bio-Based High-Performance Polymers for Sustainable Packaging. Polymers 2021, 13, 2460. https://doi.org/10.3390/polym13152460
Guidotti G, Soccio M, Gazzano M, Siracusa V, Lotti N. Poly(Alkylene 2,5-Thiophenedicarboxylate) Polyesters: A New Class of Bio-Based High-Performance Polymers for Sustainable Packaging. Polymers. 2021; 13(15):2460. https://doi.org/10.3390/polym13152460
Chicago/Turabian StyleGuidotti, Giulia, Michelina Soccio, Massimo Gazzano, Valentina Siracusa, and Nadia Lotti. 2021. "Poly(Alkylene 2,5-Thiophenedicarboxylate) Polyesters: A New Class of Bio-Based High-Performance Polymers for Sustainable Packaging" Polymers 13, no. 15: 2460. https://doi.org/10.3390/polym13152460
APA StyleGuidotti, G., Soccio, M., Gazzano, M., Siracusa, V., & Lotti, N. (2021). Poly(Alkylene 2,5-Thiophenedicarboxylate) Polyesters: A New Class of Bio-Based High-Performance Polymers for Sustainable Packaging. Polymers, 13(15), 2460. https://doi.org/10.3390/polym13152460