Halogen-Free Flame Retardant Polypropylene Fibers with Modified Intumescent Flame Retardant: Preparation, Characterization, Properties and Mode of Action
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Modification of the Dohor-6000A
2.3. Preparation of Halogen-Free Flame Retardant Polypropylene Chips
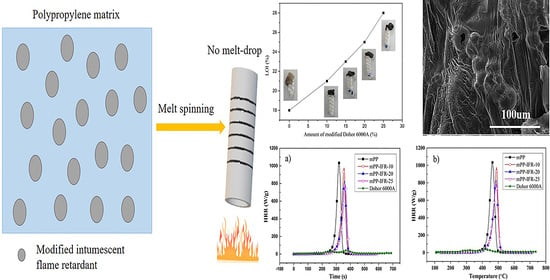
2.4. Melt Spinning Halogen-Free Flame Retardant Polypropylene Chips
2.5. Characterization
3. Results and Discussion
3.1. Rheological Behavior of the Flame Retardant Polypropylene Resins
3.2. Structure and Properties of the Flame Retardant Polypropylene Fibers
3.2.1. Aggregation Structure
3.2.2. Morphology
3.2.3. Mechanical Properties
3.3. Flame Retardant Performance and the Mode of Action for the Flame Retardant
3.3.1. Flame Retardancy
3.3.2. Analysis of Char Residues
3.3.3. Pyrolysis Analysis
3.3.4. The Mode of Action for the Modified Dohor-6000A
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Broda, J.; Fabia, J.; Bączek, M.; Ślusarczyk, C. Supramolecular Structure of Polypropylene Fibers Extruded with Addition of Functionalized Reduced Graphene Oxide. Polymers 2020, 12, 910. [Google Scholar] [CrossRef]
- Qian, Y.; Wei, P.; Jiang, P.; Zhao, X.; Yu, H. Synthesis of a novel hybrid synergistic flame retardant and its application in PP/IFR. Polym. Degrad. Stab. 2011, 96, 1134–1140. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, S.; Sun, J.; Gu, X. Flame Retardancy and Thermal Stability of Polypropylene Composite Containing Ammonium Sulfamate Intercalated Kaolinite. Ind. Eng. Chem. Res. 2016, 55, 7669–7678. [Google Scholar] [CrossRef]
- Li, Y.-L.; Kuan, C.-F.; Hsu, S.-W.; Chen, C.-H.; Kuan, H.; Lee, F.-M.; Yip, M.-C.; Chiang, C.-L. Preparation, thermal stability and flame-retardant properties of halogen-free polypropylene composites. High Perform. Polym. 2012, 24, 478–487. [Google Scholar] [CrossRef]
- Doğan, M.; Bayramlı, E. Effect of boron phosphate on the mechanical, thermal and fire retardant properties of polypropylene and polyamide-6 fibers. Fiber Polym. 2013, 14, 1595–1601. [Google Scholar] [CrossRef]
- Kuvshinnikova, O.I.; Lee, R.E.; Favstritsky, N. Guarding against bloom in FR/UV polypropylene fiber. J. Vinyl Addit. Technol. 2000, 6, 109–112. [Google Scholar] [CrossRef]
- Salaun, F.; Creach, G.; Rault, F.; Almeras, X. Thermo-physical properties of polypropylene fibers containing a microencapsulated flame retardant. Polym. Adv. Technol. 2012, 24, 236–248. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, Y.; Zhang, L.; Wang, H. Synergistic effect of expandable graphite and intumescent flame retardants on the flame retardancy and thermal stability of polypropylene. J. Mater. Sci. 2016, 51, 5857–5871. [Google Scholar] [CrossRef]
- Xiao, D.; Li, Z.; De Juan, S.; Gohs, U.; Wagenknecht, U.; Voit, B.; Wang, D.-Y. Preparation, fire behavior and thermal stability of a novel flame retardant polypropylene system. J. Therm. Anal. Calorim. 2016, 125, 321–329. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, J.; Wang, Q.; Wilkie, C.A.; O’Hare, D. Flame retardant polymer/layered double hydroxide nanocomposites. J. Mater. Chem. A 2014, 2, 10996–11016. [Google Scholar] [CrossRef]
- Liu, X.; Gu, X.; Zhang, S.; Jiang, Y.; Sun, J.; Dong, M. Effects of dihydrogen phosphate intercalated layered double hydroxides on the crystal behaviors and flammability of polypropylene. J. Appl. Polym. Sci. 2013, 130, 3645–3651. [Google Scholar] [CrossRef]
- Shao, Z.-B.; Deng, C.; Tan, Y.; Yu, L.; Chen, M.-J.; Chen, L.; Wang, Y.-Z. Ammonium polyphosphate chemically-modified with ethanolamine as an efficient intumescent flame retardant for polypropylene. J. Mater. Chem. A 2014, 2, 13955–13965. [Google Scholar] [CrossRef]
- Yuan, G.; Yang, B.; Chen, Y.; Jia, Y. Synthesis of a novel multi-structure synergistic POSS-GO-DOPO ternary graft flame retardant and its application in polypropylene. Compos. Part A 2019, 117, 345–356. [Google Scholar] [CrossRef]
- Zhang, S.; Horrocks, A.R. A review of flame retardant polypropylene fibres. Prog. Polym. Sci. 2003, 28, 1517–1538. [Google Scholar] [CrossRef]
- Lv, P.; Wang, Z.; Hu, K.; Fan, W. Flammability and thermal degradation of flame retarded polypropylene composites containing melamine phosphate and pentaerythritol derivatives. Polym. Degrad. Stab. 2005, 90, 523–534. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Fage, J.; Liang, S.; Gaan, S. An Overview of Mode of Action and Analytical Methods for Evaluation of Gas Phase Activities of Flame Retardants. Polymers 2015, 7, 504–526. [Google Scholar] [CrossRef] [Green Version]
- Darnerud, P.D. Brominated Flame Retardants as Possible Endocrine Distrupters. Int. J. Androl. 2008, 31, 152. [Google Scholar] [CrossRef]
- Howell, B.A.; Daniel, Y.G. Incorporation of Comonomer exo-5-(Diphenylphosphato)Isosorbide-2-endo-Acrylate to Generate Flame Retardant Poly(Styrene). Polymers 2019, 11, 2038. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Tian, B.; Zhu, Q.; Duan, C. Characterization and Application of PVDF and Its Copolymer Films Prepared by Spin-Coating and Langmuir–Blodgett Method. Polymers 2019, 11, 2033. [Google Scholar] [CrossRef] [Green Version]
- Shaw, S.D.; Blum, A.; Weber, R.; Kannan, K.; Rich, D.; Lucas, D.; Koshland, C.P.; Dobraca, D.; Hanson, S.; Birnbaum, L.S. Halogenated Flame Retardants. Do the Fire Safety Benefits Justify the Risks? Rev. Environ. Health 2010, 25, 261–305. [Google Scholar] [CrossRef]
- Alace, M.; Wenning, R.J. The Significance of Brominated Flame Retardants in the Environment: Current Understanding, Issues and Challenges. Chemosphere 2002, 46, 579–582. [Google Scholar]
- Nie, S.; Peng, C.; Yuan, S.; Zhang, M. Thermal and flame retardant properties of novel intumescent flame retardant polypropylene composites. J. Therm. Anal. Calorim. 2013, 113, 865–871. [Google Scholar] [CrossRef]
- Tsuyumoto, I.; Miura, Y.; Nirei, M.; Ikurumi, S.; Kumagai, T. Highly flame retardant coating consisting of starch and amorphous sodium polyborate. J. Mater. Sci. 2011, 46, 5371–5377. [Google Scholar] [CrossRef]
- Tsai, K.-C.; Kuan, H.-C.; Chou, H.-W.; Kuan, C.-F.; Chen, C.-H.; Chiang, C.-L. Preparation of expandable graphite using a hydrothermal method and flame-retardant properties of its halogen-free flame-retardant HDPE composites. J. Polym. Res. 2010, 18, 483–488. [Google Scholar] [CrossRef]
- Takashi, K.; Eric, G.; Jenny, H.; Richard, H.; Walid, A.; Jack, D. Thermal degradation and flammability properties of poly(propylene)/carbon nanotube composites. Macromol. Rapid Commun. 2002, 23, 761–765. [Google Scholar]
- Horrocks, A.; Kandola, B.; Davies, P.; Zhang, S.; Padbury, S. Developments in flame retardant textiles—A review. Polym. Degrad. Stab. 2005, 88, 3–12. [Google Scholar] [CrossRef]
- Zhang, S.; Horrocks, A.R.; Hull, R.; Kandola, B. Flammability, degradation and structural characterization of fibre-forming polypropylene containing nanoclay–flame retardant combinations. Polym. Degrad. Stab. 2006, 91, 719–725. [Google Scholar] [CrossRef]
- Huang, H.; Tian, M.; Liu, L.; Liang, W.; Zhang, L. Effect of particle size on flame retardancy of Mg(OH)2-filled ethylene vinyl acetate copolymer composites. J. Appl. Polym. Sci. 2006, 100, 4461–4469. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Duquesne, S.; Rochery, M. Recent Advances for Intumescent Polymers. Macromol. Mater. Eng. 2004, 289, 499–511. [Google Scholar] [CrossRef]
- Rault, F.; Giraud, S.; Salaün, F.; Almeras, X. Development of a Halogen Free Flame Retardant Masterbatch for Polypropylene Fibers. Polymers 2015, 7, 220–234. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Song, L.; Tai, Q.; Wang, X.; Yu, B.; Yuan, Y.; Hu, Y.; Yuen, K.K.R. Comparative study on the flame retarded efficiency of melamine phosphate, melamine phosphite and melamine hypophosphite on poly(butylene succinate) composites. Polym. Degrad. Stab. 2014, 105, 248–256. [Google Scholar] [CrossRef]
- Liu, X.; Gu, X.; Sun, J.; Zhang, S. Preparation and characterization of chitosan derivatives and their application as flame retardants in thermoplastic polyurethane. Carbohydr. Polym. 2017, 167, 356–363. [Google Scholar] [CrossRef]
- Focke, W.W.; Labuschagne, F.J.W.; Strydom, C.A. Strong base catalyzed intumescent flame retardants. J. Mater. Sci. Lett. 2000, 19, 617–618. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, H.; Wang, T.; Yao, M.; Xie, J.; Jiao, Y. Flame-retardant effect of cross-linked phosphazene derivatives and pentaerythritol derivatives on polypropylene. J. Therm. Anal. Calorim. 2020, 212. [Google Scholar] [CrossRef]
- Shao, Z.-B.; Deng, C.; Tan, Y.; Chen, M.-J.; Chen, L.; Wang, Y.-Z. Flame retardation of polypropylene via a novel intumescent flame retardant: Ethylenediamine-modified ammonium polyphosphate. Polym. Degrad. Stab. 2014, 106, 88–96. [Google Scholar] [CrossRef]
- Zhang, C.; Li, G.-Q.; Wang, Y.-C. Probabilistic analysis of steel columns protected by intumescent coatings subjected to natural fires. Struct. Saf. 2014, 50, 16–26. [Google Scholar] [CrossRef]
- Liang, B.; Hong, X.; Zhu, M.; Gao, C.; Wang, C.; Tsubaki, N. Synthesis of novel intumescent flame retardant containing phosphorus, nitrogen and boron and its application in polyethylene. Polym. Bull. 2015, 72, 2967–2978. [Google Scholar] [CrossRef]
- Qi, J.; Wen, Q.; Zhu, J.; He, T. Synthesis of a novel intumescent flame retardant based on phosphorus, nitrogen, and silicone, and application in VMQ. J. Therm. Anal. Calorim. 2019, 137, 1549–1557. [Google Scholar] [CrossRef]
- Schartel, B.; Perret, B.; Dittrich, B.; Ciesielski, M.; Krämer, J.; Müller, P.; Altstädt, V.; Zang, L.; Döring, M. Flame Retardancy of Polymers: The Role of Specific Reactions in the Condensed Phase. Macromol. Mater. Eng. 2016, 301, 9–35. [Google Scholar] [CrossRef]
- Camino, G.; Costa, L.; Trossarelli, L. Study of the mechanism of intumescence in fire retardant polymers: Part I—Thermal degradation of ammonium polyphosphate-pentaerythritol mixtures. Polym. Degrad. Stab. 1984, 6, 243–252. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Y.; Li, Y.; Shao, Q.; Yan, X.; Han, C.; Wang, Z.; Liu, Z.; Guo, Z. Flame-retardant rigid polyurethane foam with a phosphorus-nitrogen single intumescent flame retardant. Polym. Adv. Technol. 2018, 29, 668–676. [Google Scholar] [CrossRef]
- Lu, Y.; Jia, Y.; Zhang, G.; Zhang, F. An eco-friendly intumescent flame retardant with high efficiency and durability for cotton fabric. Cellulose 2018, 25, 5389–5404. [Google Scholar] [CrossRef]
- Chen, P.-F.; Yan, K.-K.; Leng, J.-X.; Zhang, F.; Jiao, L. Synergistic smoke suppression effect of epoxy cross-linked structure and ferrocene on epoxy-based intumescent flame-retardant coating. Plast. Rubber Compos. 2018, 47, 258–265. [Google Scholar] [CrossRef]
- Dai, P.-B.; Wang, X.-L.; Wang, D.-Y.; Chen, L.; Wang, Y.-Z. Effect of Modified Intumescent Flame Retardant via Surfactant/Polyacrylate Latex on Properties of Intumescent Flame Retardant ABS Composites. J. Macromol. Sci. Part B 2008, 47, 1087–1095. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, Z.; Zhang, Q.; Jiang, M.; Zhong, Z.; Chen, T.; Jiang, J. Modified montmorillonite combined with intumescent flame retardants on the flame retardancy and thermal stability properties of unsaturated polyester resins. Polym. Adv. Technol. 2019, 30, 998–1009. [Google Scholar] [CrossRef]
- Velasco, J.; Morhain, C.; Martínez, A.; Perez, M.A.R.; De Saja, J. The effect of filler type, morphology and coating on the anisotropy and microstructure heterogeneity of injection-moulded discs of polypropylene filled with aluminium and magnesium hydroxides. Part 1. A wide-angle X-ray diffraction study. Polymers 2002, 43, 6805–6811. [Google Scholar] [CrossRef]
- Urman, K.; Otaigbe, J.U. New phosphate glass/polymer hybrids—Current status and future prospects. Prog. Polym. Sci. 2007, 32, 1462–1498. [Google Scholar] [CrossRef]
- Wang, P.; Cai, Z. Highly efficient flame-retardant epoxy resin with a novel DOPO-based triazole compound: Thermal stability, flame retardancy and mechanism. Polym. Degrad. Stab. 2017, 137, 138–150. [Google Scholar] [CrossRef]
- Cullis, F.C.; Hirschler, M.M. The significance of thermoanalytical measurements in the assessment of polymer flammability. Polymer 1983, 24, 834–840. [Google Scholar] [CrossRef]











| Sample Code | Mass Content of Polypropylene/% | Mass Content of Surface Modified Dohor-6000A/% | Mass Content of MA-g-Polypropylene/% |
|---|---|---|---|
| mPP | 95 | 0 | 5 |
| mPP-IFR-10 | 85 | 10 | 5 |
| mPP-IFR-15 | 80 | 15 | 5 |
| mPP-IFR-20 | 75 | 20 | 5 |
| mPP-IFR-25 | 70 | 25 | 5 |
| mPP-IFR-30 | 65 | 30 | 5 |
| PP-IFR-30 | 70 | 30 | 0 |
| Sample | Tm/°C | Tc/°C | ΔHc/-J g−1 | W1/2/°C | Xc/% |
|---|---|---|---|---|---|
| mPP | 164.8 | 112.4 | 64.23 | 5.3 | 33.4 |
| mPP-IFR-10 | 166.1 | 116.5 | 85.38 | 4.0 | 47.36 |
| mPP-IFR-15 | 164.6 | 117.6 | 79.22 | 3.9 | 45.10 |
| mPP-IFR-20 | 166.3 | 118.4 | 73.27 | 4.2 | 42.46 |
| mPP-IFR-25 | 166.7 | 120.3 | 71.04 | 4.2 | 44.6 |
| mPP-IFR-30 | 165.3 | 119.7 | 66.61 | 4.5 | 43.40 |
| Sample | The Peak Width at Half Maximum (H) | Average Orientation Degree of the Crystal Region (f) |
|---|---|---|
| mPP | 11.16 | 0.938 |
| mPP-IFR-10 | 10.58 | 0.941 |
| mPP-IFR-20 | 10.65 | 0.940 |
| mPP-IFR-30 | 11.95 | 0.934 |
| Sample | Draw Ratio | Linear Density/Dtex | Breaking Strength/cN dtex−1 | Elongation/% |
|---|---|---|---|---|
| mPP | 3.4 | 8.45 | 5.03 | 60 |
| mPP-IFR-10 | 3.4 | 8.5 | 4.31 | 28 |
| mPP-IFR-15 | 3.4 | 8.71 | 3.83 | 32.95 |
| mPP-IFR-20 | 3.4 | 9.10 | 3.59 | 25.35 |
| mPP-IFR-25 | 3.4 | 8.76 | 3.35 | 25.45 |
| mPP-IFR-30 | 3.4 | 9.02 | 2.9 | 26.6 |
| Sample | pHRR/W g−1 | HRC/J g−1 k−1 | THR/kJ g−1 | TpHRR/°C |
|---|---|---|---|---|
| mPP | 1042 | 1034 | 39.4 | 462 |
| mPP-IFR-10 | 976 | 980 | 37.7 | 490 |
| mPP-IFR-20 | 831 | 840 | 35.0 | 487 |
| mPP-IFR-25 | 771 | 801 | 33.1 | 487 |
| modified Dohor-6000A | 34.06 | 40 | 5.0 | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Wu, L.; Yan, X.; Zhang, S.; Dong, L.; Su, Z.; Zhong, T.; Jiang, C.; Chen, Y.; Jiang, M.; et al. Halogen-Free Flame Retardant Polypropylene Fibers with Modified Intumescent Flame Retardant: Preparation, Characterization, Properties and Mode of Action. Polymers 2021, 13, 2553. https://doi.org/10.3390/polym13152553
Xu Q, Wu L, Yan X, Zhang S, Dong L, Su Z, Zhong T, Jiang C, Chen Y, Jiang M, et al. Halogen-Free Flame Retardant Polypropylene Fibers with Modified Intumescent Flame Retardant: Preparation, Characterization, Properties and Mode of Action. Polymers. 2021; 13(15):2553. https://doi.org/10.3390/polym13152553
Chicago/Turabian StyleXu, Qibin, Lei Wu, Xiang Yan, Shengchang Zhang, Linan Dong, Zexi Su, Tianhaoyue Zhong, Chunhui Jiang, Yuan Chen, Mengjin Jiang, and et al. 2021. "Halogen-Free Flame Retardant Polypropylene Fibers with Modified Intumescent Flame Retardant: Preparation, Characterization, Properties and Mode of Action" Polymers 13, no. 15: 2553. https://doi.org/10.3390/polym13152553
APA StyleXu, Q., Wu, L., Yan, X., Zhang, S., Dong, L., Su, Z., Zhong, T., Jiang, C., Chen, Y., Jiang, M., & Liu, P. (2021). Halogen-Free Flame Retardant Polypropylene Fibers with Modified Intumescent Flame Retardant: Preparation, Characterization, Properties and Mode of Action. Polymers, 13(15), 2553. https://doi.org/10.3390/polym13152553