Clinical Efficacy of Polycaprolactone β-Calcium Triphosphate Composite for Osteoconduction in Rabbit Bone Defect Model
Abstract
:1. Introduction
2. Materials and Methods
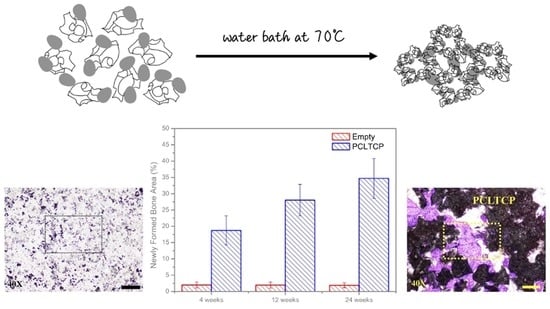
2.1. Preparation of PCL–TCP Composites
2.2. Physicochemical Analysis
2.3. Setting Time and Static Compression Test
2.4. Surgical Procedures and Implantation
2.5. Radiological and Histomorphometric Analyses
2.6. Statistical Analysis
3. Results
3.1. Morphology and Mechanical Analysis
3.2. Clinical Observation and Gross Bone Morphology
3.3. Radiographic Analyses
3.4. Histomorphometric Examinations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chapekar, M.S. Tissue engineering: Challenges and opportunities. J. Biomed. Mater. Res. 2000, 53, 617–620. [Google Scholar] [CrossRef]
- Einhorn, T.A. The cell and molecular biology of fracture healing. Clin. Orthop. Relat. Res. 1998. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunatillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur Cell Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef]
- Williams, C.G.; Malik, A.N.; Kim, T.K. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 2005, 26, 1211–1218. [Google Scholar] [CrossRef]
- Glenske, K.; Donkiewicz, P.; Köwitsch, A. Applications of metals for bone regeneration. Int. J. Mol. Sci. 2018, 19, 826. [Google Scholar] [CrossRef] [Green Version]
- Pobloth, A.M.; Checa, S.; Razi, H. Mechanobiologically optimized 3D titanium-mesh scaffolds enhance bone regeneration in critical segmental defects in sheep. Sci. Transl. Med. 2018. [Google Scholar] [CrossRef] [Green Version]
- LeGeros, R.Z. Biodegradation and bioresorption of calcium phosphate ceramics. Clin. Mater. 1993, 14, 65–88. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Heymann, D.; Bouler, J.M. Osteoclastic resorption of calcium phosphate ceramics with different hydroxyapatite/β-tricalcium phosphate ratios. Biomaterials 1997, 18, 1037–1041. [Google Scholar] [CrossRef]
- Kung, F.C.; Lin, C.C.; Lai, W.F.T. Osteogenesis of human adipose-derived stem cells on hydroxyapatite-mineralized poly (lactic acid) nanofiber sheets. Mater. Sci. Eng. C 2014, 45, 578–588. [Google Scholar] [CrossRef]
- Shim, J.H.; Yoon, M.C.; Jeong, C.M. Efficacy of rhBMP-2 loaded PCL/PLGA/β-TCP guided bone regeneration membrane fabricated by 3D printing technology for reconstruction of calvaria defects in rabbit. Biomed. Mater. 2014, 9, 065006. [Google Scholar] [CrossRef]
- Hsu, S.; Hsieh, C.T.; Sun, Y.M. Synthesis and characterization of waterborne polyurethane containing poly (3-hydroxybutyrate) as new biodegradable elastomers. J. Mater. Chem. 2015, 3, 9089–9097. [Google Scholar] [CrossRef]
- Zhang, E.; Zhu, C.; Yang, J. Electrospun PDLLA/PLGA composite membranes for potential application in guided tissue regeneration. Mater. Sci. Eng. C 2016, 58, 278–285. [Google Scholar] [CrossRef]
- Cooke, S.L.; Whittington, A.R. Influence of therapeutic radiation on polycaprolactone and polyurethane biomaterials. Mater. Sci. Eng. C 2016, 60, 78–83. [Google Scholar] [CrossRef]
- Lee, S.; Choi, D.; Shim, J.H. Efficacy of three-dimensionally printed polycaprolactone/beta tricalcium phosphate scaffold on mandibular reconstruction. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Horch, H.H.; Sader, R.; Pautke, C. Synthetic, pure-phase beta-tricalcium phosphate ceramic granules (Cerasorb®) for bone regeneration in the reconstructive surgery of the jaws. Int. J. Oral. Maxill. Surg. 2006, 35, 708–713. [Google Scholar] [CrossRef]
- Liu, S.; Jin, F.; Lin, K. The effect of calcium silicate on in vitro physiochemical properties and in vivo osteogenesis, degradability and bioactivity of porous β-tricalcium phosphate bioceramics. Biomed. Mater. 2013, 8, 025008. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.A.; Calvo-Guirado, J.L.; Romanos, G.E. Critical size defects for bone regeneration experiments in rabbit calvariae: Systematic review and quality evaluation using ARRIVE guidelines. Clin. Oral Implant. Res. 2015, 26, 915–930. [Google Scholar] [CrossRef]
- Huang, S.H.; Hsu, T.T.; Huang, T.H. Fabrication and characterization of polycaprolactone and tricalcium phosphate composites for tissue engineering applications. J. Dent. Sci. 2017, 12, 33–43. [Google Scholar] [CrossRef]
- Ramay, H.R.R.; Zhang, M. Biphasic calcium phosphate nanocomposite porous scaffolds for load-bearing bone tissue engineering. Biomaterials 2004, 25, 5171–5180. [Google Scholar] [CrossRef]
- Ma, C.; Ma, Z.; Yang, F. Poly (propylene fumarate)/β-calcium phosphate composites for enhanced bone repair. Biomed. Mater. 2019, 14, 045002. [Google Scholar] [CrossRef]
- Huang, B.; Caetano, G.; Vyas, C.; Blaker, J.J.; Diver, C.; Bártolo, P. Polymer-ceramic composite scaffolds: The effect of hydroxyapatite and β-tri-calcium phosphate. Materials 2018, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H.; Lee, M.Y.; Shyu, V.B.; Chen, Y.C.; Chen, C.T.; Chen, J.P. Surface modification of polycaprolactone scaffolds fabricated via selective laser sintering for cartilage tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 40, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Hannink, G.; Arts, J.J.C. Bioresorbability, porosity and mechanical strength of bone substitutes: What is optimal for bone regeneration? Injury 2011, 42, S22–S25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, C.H.; Chen, Y.W.; Shie, M.Y. Poly (dopamine)-assisted immobilization of Xu Duan on 3D printed poly (lactic acid) scaffolds to up-regulate osteogenic and angiogenic markers of bone marrow stem cells. Materials 2015, 8, 4299–4315. [Google Scholar] [CrossRef]
- Liu, F.; Liu, C.; Zheng, B.; He, J.; Liu, J.; Chen, C.; Lee, I.S.; Wang, X.; Liu, Y. Synergistic Effects on Incorporation of beta-Tricalcium Phosphate and Graphene Oxide Nanoparticles to Silk Fibroin/Soy Protein Isolate Scaffolds for Bone Tissue Engineering. Polymers 2020, 12, 69. [Google Scholar] [CrossRef] [Green Version]
- Fisher, J.; Vehof, J.; Dean, D.; van der Waerden, J.; Holland, T.; Mikos, A. Soft and hard tissue response to photocross-linked poly(propylene fumarate) scaffolds in a rabbit model. J. Biomed. Mater. Res. 2002, 59, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, Y.; Jin, Q.; Kikuchi, M.; Mamood, J.; Takita, H. Geometry of artificial ECM: Sizes of pores controlling phenotype expression in BMP-induced osteogenesis and chondrogenesis. Connect. Tissue Res. 2002, 43, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Kujala, S.; Ryhanen, J.; Danilov, A.; Tuukkanen, J. Effect of porosity on the osteointe-gration and bone ingrowth of a weight-bearing nickel-titanium bone graft substitute. Biomaterials 2003, 24, 4691–4697. [Google Scholar] [CrossRef]
- Rai, B.; Ho, K.H.; Lei, Y.; Si-Hoe, K.M.; Teo, C.M.J.; Yacob, K.B.; Chen, F.; Ng, F.C.; Teoh, S.H. Polycaprolactone-20% tricalcium phosphate scaffolds in combination with platelet-rich plasma for the treatment of critical-sized defects of the mandible: A pilot study. J. Oral Maxillofac. Surg. 2007, 65, 2195–2205. [Google Scholar] [CrossRef]





| Group | No. | Treatment | Implantation Time (Week) | |
|---|---|---|---|---|
| Left | Right | |||
| A | 1 | PCLTCP | β-TCP | 4 |
| 2 | β-TCP | PCLTCP | 4 | |
| 3 | PCLTCP | Empty | 4 | |
| 4 | β-TCP | Empty | 4 | |
| 5 | Empty | PCLTCP | 4 | |
| 6 | Empty | β-TCP | 4 | |
| B | 7 | PCLTCP | β-TCP | 12 |
| 8 | β-TCP | PCLTCP | 12 | |
| 9 | PCLTCP | Empty | 12 | |
| 10 | β-TCP | Empty | 12 | |
| 11 | Empty | PCLTCP | 12 | |
| 12 | Empty | β-TCP | 12 | |
| C | 13 | PCLTCP | β-TCP | 24 |
| 14 | β-TCP | PCLTCP | 24 | |
| 15 | PCLTCP | Empty | 24 | |
| 16 | β-TCP | Empty | 24 | |
| 17 | Empty | PCLTCP | 24 | |
| 18 | Empty | β-TCP | 24 | |
| PCL-TCP | β-TCP | |
|---|---|---|
| Product name | “Wiltrom” Bitrans Bone Graft Substitute | “Wiltrom” Osteocera Bone Graft Substitute |
| Composite | β-tricalcium phosphate, β-TCP 75% polycaprolactone, PCL 25% | β-tricalcium phosphate, β-TCP > 95% |
| Pore size From SEM | 235.28 ± 113.50 (μm) | 463 ± 88.75 (μm) |
| Porosity From SEM | 43.00% ± 15.98% | 83.05% ± 1.53% |
| Compressive strength | 15.104 ± 0.530 (MPa) | 0.85 ± 0.19 (MPa) |
| Setting time | 72.6 ± 8.5 (s) | NA |
| Group/Treatment | Remaining Implant (%) | Void Space Area (%) | Newly Formed Bone Area (%) | |
|---|---|---|---|---|
| A | PCLTCP | 50.22 ± 7.61 | 31.06 ± 7.36 | 18.72 ± 4.48 |
| β-TCP | 51.73 ± 8.22 | 26.57 ± 6.92 | 21.70 ± 7.44 | |
| Empty | NA | 98.00 ± 0.92 | 2.00 ± 0.92 | |
| B | PCLTCP | 53.72 ± 4.61 | 18.25 ± 5.66 | 28.03 ± 4.82 * |
| β-TCP | 32.94 ± 6.96 * | 35.41 ± 10.72 | 31.32 ± 5.91 * | |
| Empty | NA | 98.05 ± 0.92 | 1.95 ± 0.92 | |
| C | PCLTCP | 36.51 ± 6.84 * | 28.80 ± 4.04 | 34.69 ± 6.06 * |
| β-TCP | 23.02 ± 7.22 * | 39.87 ± 11.14 | 37.11 ± 10.65 * | |
| Empty | NA | 98.10 ± 0.76 | 1.90 ± 0.76 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-M.; Chen, S.-M.; Lin, S.-F.; Liang, H.-C.; Wu, C.-C. Clinical Efficacy of Polycaprolactone β-Calcium Triphosphate Composite for Osteoconduction in Rabbit Bone Defect Model. Polymers 2021, 13, 2552. https://doi.org/10.3390/polym13152552
Chen C-M, Chen S-M, Lin S-F, Liang H-C, Wu C-C. Clinical Efficacy of Polycaprolactone β-Calcium Triphosphate Composite for Osteoconduction in Rabbit Bone Defect Model. Polymers. 2021; 13(15):2552. https://doi.org/10.3390/polym13152552
Chicago/Turabian StyleChen, Chiu-Ming, Shen-Mao Chen, Shiou-Fu Lin, Huang-Chien Liang, and Chia-Chun Wu. 2021. "Clinical Efficacy of Polycaprolactone β-Calcium Triphosphate Composite for Osteoconduction in Rabbit Bone Defect Model" Polymers 13, no. 15: 2552. https://doi.org/10.3390/polym13152552
APA StyleChen, C. -M., Chen, S. -M., Lin, S. -F., Liang, H. -C., & Wu, C. -C. (2021). Clinical Efficacy of Polycaprolactone β-Calcium Triphosphate Composite for Osteoconduction in Rabbit Bone Defect Model. Polymers, 13(15), 2552. https://doi.org/10.3390/polym13152552