2.1. Materials
Bisphenol-A (BPA; 99.0%), diphenyl carbonate (DPC; 99.0%), and potassium hydroxide (KOH; 95%) were provided by Aladdin. Anhydrous alcohol (CH3CH2OH; 99.7%), dichloromethane (CH2Cl2; 99.5%), chloroform (CHCl3; 99.0%), and n-hexane (C6H14; 97%) were purchased from Shanghai Titan. DPC was purified by recrystallization in anhydrous ethanol three times.
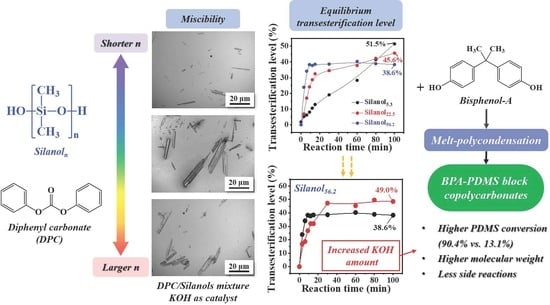
Silanol-terminated PDMS with an average chain length of 5.3 and 22.5 was supplied by Shenzhen Ji-Peng Silicone Fluoride Materials Co. (Shenzhen, China) and denoted as Silanol
5.3 and Silanol
22.5, respectively. Another silanol-terminated PDMS with an average chain length of 56.2, denoted as Silanol
56.2, was provided by ThermoFisher Scientific (Shanghai, China). The average chain length of PDMS was determined by
1H-NMR as described below. The chemical structures of BPA, PDMS, and DPC are shown in
Figure 1.
2.3. Synthesis of BPA/PDMS Copolycarbonates
Different amounts of Silanol5.3 were introduced into BPA and DPC mixtures. The molar ratio of DPC/BPA was set at 1.06, and the BPA/PDMS molar ratio was changed to 100/0, 99/1, 98/2, 96/4, 94/6, 92/8, and 90/10. The total amount of monomer was 77 mmol. A KOH amount of 100 ppm to diols on a mole basis was used. Whole reactants were placed in a 100 mL three-neck round-bottom flask equipped with a mechanical stirrer and high-vacuum bearing. The temperature was increased to 180 °C with mechanical stirring under N2 atmosphere. The material was allowed to melt for 10 min, and then the temperature was kept constant for 15 min to make the transesterification reach equilibrium. Then, the temperature was increased to 210 °C, and the pressure was gradually reduced to 120 mmHg. The temperature was held for 45 min to distill off phenol. Subsequently, the temperature was raised to 270 °C over the next 15 min, after which the pressure was reduced to 30 mmHg. After further reaction for 10 min, the final temperature was set at 280 °C, and the pressure was reduced to less than 1 mmHg. This condition was maintained for 10 min to complete the reaction. The synthesized polymer was cooled to room temperature, dissolved in dichloromethane, and precipitated in an appropriate amount of n-hexane accompanied by ultrasonic cleaning for 1 h to remove unreacted PDMS. The final product was collected by vacuum filtration and dried in a vacuum oven at 100 °C for 24 h until their use for characterizations.
A similar procedure was performed using Silanol22.5 and Silanol56.2 as raw materials. The molar ratio of DPC to BPA was kept at 1.06, and the amount of PDMS was set at 6 mol% BPA.
2.4. Characterizations
ATR-FTIR experiments were performed on a Nicolet 5700 spectrometer with a DTGS detector in the range of 400–4000 cm–1 at a resolution of 4 cm−1. The samples were dissolved in dichloromethane at a concentration of 5 wt%, and then 50 μL of solution was spread on a KBr pellet. The solvent was removed by vacuum desiccation at room temperature before ATR-FTIR analysis.
1H,
13C, and
29Si NMR spectroscopic measurements were conducted using a Bruker DMX-600 NMR spectrometer. CDCl
3 with TMS served as deuterium solvents. The incorporated ratios, block contents, and conversions of PDMS were designated as
NPDMS,
WPDMS, and Conversion, respectively. They were measured by
where [
a] represents an integrated value of a methyl group in a dimethylsiloxane moiety observed at around δ −0.06 to 0.3, [
b] represents an integrated value of a methyl group in a BPA moiety observed at around δ 1.5 to 1.9, and
N0 represents the initial feed amount of PDMS.
The molecular weights and molecular-weight distributions of silanols were determined by gel permeation chromatography (GPC). The measurements were performed using a Waters 1515 HPLC pump equipped with a refractive-index detector (Waters 2414). Tetrahydrofuran (THF) was used as the mobile phase at a flow rate of 1 mL/min at 35 °C. Weight-average molecular weight (Mw) and polydispersity index (PDI) were determined against linear polystyrene standards.
The viscosity-average molecular weight (
Mη) of the polycondensation product was determined with an Ubbelohde viscometer at 25 ± 0.5 °C using CHCl
3 as the solvent and was calculated by Equations (4) and (5) as follows [
29]:
where
C is the concentration of the solution (always 0.01 g mL
−1),
ηr is the relative viscosity, and
ηsp is the specific viscosity. According to ref. [
29], the characteristic parameters
K and α of BPA-PC are 0.0111 mL g
−1 and 0.82, respectively.
Similarly, the
Mη of PDMS can also be determined using an Ubbelohde viscometer. When CHCl
3 was replaced with toluene as the solvent, it can be described as follows:
Color difference (Δ
C), which represents the yellowness of the synthesized product, was measured with an ultraviolet spectrophotometer (UV-
vis; UV1900, Youke Instrument). A CHCl
3 solution with a concentration of 0.01 g mL
−1 was used, and Δ
C was determined by:
where
T445,
T555, and
T600 represent the transmittance of the solution relative to CHCl
3 at wavelengths of 445, 555, and 600 nm. Furthermore,
T =
T445 +
T555 +
T600.
The morphology of transesterification products was observed using an OM system (Olympus CX21). We took a drop of transesterification product onto a glass slide, gently pressed the sample to flow naturally, and used a low-power zoom lens (40 × 10) to observe the changes in the morphology of the two phases.
The glass-transition temperature (Tg) of PC was measured with a differential scanning calorimetry (DSC) system (DSC25, TA Instruments) within 20 °C to 250 °C at a heating rate of 10 °C min−1 in N2 atmosphere. To eliminate the thermal history of the samples, Tg was determined as the inflection point of the transition during the second heating process.
The decomposition temperature was tested using a thermogravimetric analysis (TGA) apparatus (Netzsch STA 409). The samples were heated from 50 °C to 800 °C at a heating rate of 10 °C min−1 under a nitrogen flow of 50 mL min−1. The 5% weight-loss temperature was recoded as Td, 5%.