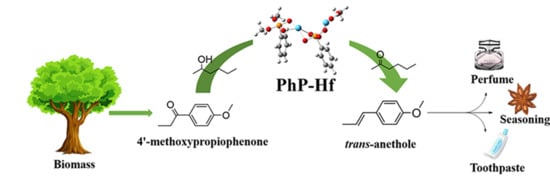
Catalytic Stereoselective Conversion of Biomass-Derived 4′-Methoxypropiophenone to Trans-Anethole with a Bifunctional and Recyclable Hf-Based Polymeric Nanocatalyst
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Catalysts Preparation
2.3. Catalytic Reaction
2.4. Characterization Methods
3. Results and Discussion
3.1. Catalyst Characterization
3.2. Activity of Different Catalysts
3.3. Effect of Reaction Temperature and Time
3.4. Catalyst Recycle Study
3.5. Reaction Mechanism Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harrison, A.; Tang, C. Amphiphilic polymer nanoreactors for multiple step, one-pot reactions and spontaneous product separation. Polymers 2021, 13, 1992. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Z. Catalytic conversion of biomass into chemicals and fuels over magnetic catalysts. ACS Catal. 2015, 6, 326–338. [Google Scholar] [CrossRef]
- Li, H.; Fang, Z.; Yang, S. Direct catalytic transformation of biomass derivatives into biofuel component gamma-valerolactone with magnetic nickel-zirconium nanoparticles. ChemPlusChem 2016, 81, 135–142. [Google Scholar] [CrossRef]
- Yang, T.; Li, H.; He, J.; Liu, Y.; Zhao, W.; Wang, Z.; Ji, X.; Yang, S. Porous Ti/Zr microspheres for efficient transfer hydrogenation of biobased ethyl levulinate to gamma-valerolactone. ACS Omega 2017, 2, 1047–1054. [Google Scholar] [CrossRef]
- Tang, X.; Zuo, M.; Li, Z.; Liu, H.; Xiong, C.; Zeng, X.; Sun, Y.; Hu, L.; Liu, S.; Lei, T.; et al. Green processing of lignocellulosic biomass and its derivatives in deep eutectic solvents. ChemSusChem 2017, 10, 2696–2706. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Zhao, W.; Yang, S. Low-temperature and solvent-free production of biomass-derived diesel-range C17 precursor via one-pot cascade acylation–alkylation over Sn4+-montmorillonite. J. Ind. Eng. Chem. 2018, 66, 325–332. [Google Scholar] [CrossRef]
- Pan, H.; Li, H.; Zhang, H.; Wang, A.; Yang, S. Acidic ionic liquid-functionalized mesoporous melamine-formaldehyde polymer as heterogeneous catalyst for biodiesel production. Fuel 2019, 239, 886–895. [Google Scholar] [CrossRef]
- Puke, M.; Godina, D.; Kirpluks, M.; Rizikovs, J.; Brazdausks, P. Residual birch wood lignocellulose after 2-furaldehyde production as a potential feedstock for obtaining fiber. Polymers 2021, 13, 1816. [Google Scholar] [CrossRef]
- Wu, W.; Li, Y.; Li, H.; Zhao, W.; Yang, S. Acid-base bifunctional Hf nanohybrids enable high selectivity in the catalytic conversion of ethyl levulinate to γ-valerolactone. Catalysts 2018, 8, 264. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhao, W.; Dai, W.; Long, J.; Watanabe, M.; Meier, S.; Saravanamurugan, S.; Yang, S.; Riisager, A. Noble metal-free upgrading of multi-unsaturated biomass derivatives at room temperature: Silyl species enable reactivity. Green Chem. 2018, 20, 5327–5335. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Li, Z.; Chen, J. Applications of lignin-derived catalysts for green synthesis. Green Energy Environ. 2019, 4, 210–244. [Google Scholar] [CrossRef]
- Tang, X.; Zeng, X.; Li, Z.; Hu, L.; Sun, Y.; Liu, S.; Lei, T.; Lin, L. Production of γ-valerolactone from lignocellulosic biomass for sustainable fuels and chemicals supply. Renew. Sustain. Energy Rev. 2014, 40, 608–620. [Google Scholar] [CrossRef]
- Kfoury, M.; Auezova, L.; Greige-Gerges, H.; Larsen, K.L.; Fourmentin, S. Release studies of trans-anethole from beta-cyclodextrin solid inclusion complexes by Multiple Headspace Extraction. Carbohydr. Polym. 2016, 151, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ma, M.; Sun, B.; Ren, F.; Chen, H.; Zhang, N.; Zhang, Y. Identification of characteristic aroma components of butter from Chinese butter hotpot seasoning. Food Chem. 2021, 338, 127838. [Google Scholar] [CrossRef]
- Hassam, M.; Taher, A.; Arnott, G.E.; Green, I.R.; Van Otterlo, W.A. Isomerization of allylbenzenes. Chem. Rev. 2015, 115, 5462–5569. [Google Scholar] [CrossRef]
- Ibrahim, N.; Moussa, A.Y. A comparative volatilomic characterization of Florence fennel from different locations: Antiviral prospects. Food Funct. 2021, 12, 1498–1515. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, J.; Tao, Y.; Fang, L.; Zhou, J.; Dai, M.; Liu, M.; Fang, Q. Biomass materials derived from anethole: Conversion and application. Polym. Chem. 2020, 11, 954–963. [Google Scholar] [CrossRef]
- Lastra-Barreira, B.; Francos, J.; Crochet, P.; Cadierno, V. Ruthenium(iv)catalysts for the selective estragole to trans-anetholeisomerization in environmentally friendly media. Green Chem. 2011, 13, 307–313. [Google Scholar] [CrossRef]
- Zhang, H.; Lim, L.C.; Zaki, M.; Jaenicke, S.; Chuah, G.K. A Dual-functional catalyst for cascade Meerwein-Pondorf-Verley reduction and dehydration of 4′-methoxypropiophenone to anethole. ChemSusChem 2018, 11, 3007–3017. [Google Scholar] [CrossRef]
- Amin, M.H.; Putla, S.; Hamid, S.B.A.; Bhargava, S.K. Understanding the role of lanthanide promoters on the structure–activity of nanosized Ni/γ-Al2O3 catalysts in carbon dioxide reforming of methane. Appl. Catal. A Gen. 2015, 492, 160–168. [Google Scholar] [CrossRef]
- Lopes, M.; Dussan, K.; Leahy, J.J.; Silva, V.T.D. Conversion of d-glucose to 5-hydroxymethylfurfural using Al2O3-promoted sulphated tin oxide as catalyst. Catal. Today 2017, 279, 233–243. [Google Scholar] [CrossRef]
- Wang, A.; Sudarsanam, P.; Xu, Y.; Zhang, H.; Li, H.; Yang, S. Functionalized magnetic nanosized materials for efficient biodiesel synthesis via acid–base/enzyme catalysis. Green Chem. 2020, 22, 2977–3012. [Google Scholar] [CrossRef]
- Li, H.; Fang, Z.; Smith, R.L.; Yang, S. Efficient valorization of biomass to biofuels with bifunctional solid catalytic materials. Prog. Energy Combust. 2016, 55, 98–194. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Z.; Hacking, J.; Heeres, H.J.; Yue, J. Selective fructose dehydration to 5-hydroxymethylfurfural from a fructose-glucose mixture over a sulfuric acid catalyst in a biphasic system: Experimental study and kinetic modelling. Chem. Eng. J. 2021, 409, 128182. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtárová, K. Terephthalic acid from waste PET: An efficient and reusable catalyst for xylose conversion into furfural. Catal. Today 2019, 324, 27–32. [Google Scholar] [CrossRef]
- Valekar, A.H.; Lee, M.; Yoon, J.W.; Kwak, J.; Hong, D.-Y.; Oh, K.-R.; Cha, G.-Y.; Kwon, Y.-U.; Jung, J.; Chang, J.-S.; et al. Catalytic transfer hydrogenation of furfural to furfuryl alcohol under mild conditions over Zr-MOFs: Exploring the role of metal node coordination and modification. ACS Catal. 2020, 10, 3720–3732. [Google Scholar] [CrossRef]
- Zhang, L.; Xi, G.; Chen, Z.; Jiang, D.; Yu, H.; Wang, X. Highly selective conversion of glucose into furfural over modified zeolites. Chem. Eng. J. 2017, 307, 868–876. [Google Scholar] [CrossRef]
- Liu, X.; Yin, B.; Zhang, W.; Yu, X.; Du, Y.; Zhao, S.; Zhang, G.; Liu, M.; Yan, H.; Abbotsi-Dogbey, M.; et al. Catalytic transfer hydrogenolysis of glycerol over heterogeneous catalysts: A short review on mechanistic studies. Chem. Rec. 2021, 21, 1792–1810. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Zhao, D.; Wang, Y.; Balu, A.M.; Len, C.; Luque, R. Continuous flow conversion of biomass-derived methyl levulinate into γ-valerolactone using functional metal organic frameworks. ACS Sustain. Chem. Eng. 2018, 6, 6746–6752. [Google Scholar] [CrossRef]
- Ye, L.; Han, Y.; Feng, J.; Lu, X. A review about GVL production from lignocellulose: Focusing on the full components utilization. Ind. Crop. Prod. 2020, 144, 112031. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W. Conversion of biomass to hydroxymethylfurfural: A review of catalytic systems and underlying mechanisms. Bioresour. Technol. 2017, 238, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.M.; Lima, S.; Neves, P.; Magalhães, A.L.; Fazio, E.; Neri, F.; Pereira, M.T.; Silva, A.F.; Silva, C.M.; Rocha, S.M.; et al. Integrated reduction and acid-catalysed conversion of furfural in alcohol medium using Zr,Al-containing ordered micro/mesoporous silicates. Appl. Catal. B 2016, 182, 485–503. [Google Scholar] [CrossRef] [Green Version]
- Mauriello, F.; Ariga-Miwa, H.; Paone, E.; Pietropaolo, R.; Takakusagi, S.; Asakura, K. Transfer hydrogenolysis of aromatic ethers promoted by the bimetallic Pd/Co catalyst. Catal. Today 2020, 357, 511–517. [Google Scholar] [CrossRef]
- Zhang, B.; Qi, Z.; Li, X.; Ji, J.; Zhang, L.; Wang, H.; Liu, X.; Li, C. Cleavage of lignin C–O bonds over a heterogeneous rhenium catalyst through hydrogen transfer reactions. Green Chem. 2019, 21, 5556–5564. [Google Scholar] [CrossRef]
- Shivhare, A.; Jampaiah, D.; Bhargava, S.K.; Lee, A.F.; Srivastava, R.; Wilson, K. Hydrogenolysis of Lignin-Derived Aromatic Ethers over Heterogeneous. ACS Sustain. Chem. Eng. 2021, 9, 3379–3407. [Google Scholar]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.-P.; Ren, T.-Z.; Yuan, Z.-Y. Insights into mesoporous metal phosphonate hybrid materials for catalysis. Catal. Sci. Technol. 2015, 5, 4258–4279. [Google Scholar] [CrossRef]
- Xie, C.; Song, J.; Zhou, B.; Hu, J.; Zhang, Z.; Zhang, P.; Jiang, Z.; Han, B. Porous Hafnium Phosphonate: Novel Heterogeneous Catalyst for Conversion of Levulinic Acid and Esters into γ-Valerolactone. ACS Sustain. Chem. Eng. 2016, 4, 6231–6236. [Google Scholar] [CrossRef]
- Lee, C.Y.; Sharma, A.; Semenya, J.; Anamoah, C.; Chapman, K.N.; Barone, V. Computational study of ortho-substituent effects on antioxidant activities of phenolic dendritic antioxidants. Antioxidants 2020, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Veliscek-Carolan, J.; Hanley, T.L.; Luca, V. Zirconium organophosphonates as high capacity, selective lanthanide sorbents. Sep. Purif. Technol. 2014, 129, 150–158. [Google Scholar] [CrossRef]
- Silbernagel, R.; Martin, C.H.; Clearfield, A. Zirconium(IV) phosphonate-phosphates as efficient ion-exchange materials. Inorg. Chem. 2016, 55, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Y.; Fang, Z.; Smith, R.L. Efficient catalytic transfer hydrogenation of biomass-based furfural to furfuryl alcohol with recycable Hf-phenylphosphonate nanohybrids. Catal. Today 2019, 319, 84–92. [Google Scholar] [CrossRef]
- Lin, X.-Z.; Yuan, Z.-Y. Synthesis of amorphous porous zirconium phosphonate materials: Tuneable from micropore to mesopore sizes. RSC Adv. 2014, 4, 32443–32450. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, W.; Roslan, I.I.; Jaenicke, S.; Chuah, G.-K. A combo Zr-HY and Al-HY zeolite catalysts for the one-pot cascade transformation of biomass-derived furfural to γ-valerolactone. J. Catal. 2019, 375, 56–67. [Google Scholar] [CrossRef]














| Catalyst | SBET (m2/g) | Vpore (cm3/g) | Dmean (nm) | Basicity (mmol/g) | Acidity (mmol/g) | Acid/Base Ratio |
|---|---|---|---|---|---|---|
| HfO2 | 24 | 0.16 | 28.8 | 0.24 | 0.16 | 0.67 |
| PhP-Hf (1:1.5) | 213 | 0.22 | 3.5 | 0.32 | 0.27 | 0.84 |
| Recovered PhP-Hf (1:1.5) | 203 | 0.19 | 3.6 | 0.35 | 0.15 | 0.71 |
| Entry | Catalyst | Conv (%) | Yield (%) | Select (%) | Ether Yield (%) | Acid-Base Content (mmol/g) | TOF (h−1) | Acid/Base Site Ratio | |
|---|---|---|---|---|---|---|---|---|---|
| Cis. | Trans. | ||||||||
| 1 | PhP-Hf (2:1) | 60.1 | 0.7 | 5.1 | 9.6 | 2.9 | 0.44 | 6.8 | 0.82 |
| 2 | PhP-Hf (1.5:1) | 79.5 | 3.3 | 47.6 | 77.9 | 13.6 | 0.49 | 8.1 | 0.82 |
| 3 | PhP-Hf (1:1) | 97.4 | 10.6 | 81.1 | 94.2 | 3 | 0.55 | 8.9 | 0.83 |
| 4 | PhP-Hf (1:1.5) | 99.8 | 9.9 | 88.2 | 98.3 | 1 | 0.59 | 8.5 | 0.84 |
| 5 | PhP-Hf (1:2) | 99.8 | 9.8 | 85.7 | 95.7 | 4 | 0.65 | 7.7 | 0.87 |
| Entry | Substrate | Product | Temp. (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| 1 |  |  | 220 | 2 | 94 |
| 2 |  |  | 220 | 4 | 92 |
| 3 |  |  | 220 | 6 | 90 |
| 4 |  |  | 120 | 2 | 97.6 |
| 5 |  |  | 160 | 6 | 85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, D.; Li, M.; Zhang, H.; Li, H. Catalytic Stereoselective Conversion of Biomass-Derived 4′-Methoxypropiophenone to Trans-Anethole with a Bifunctional and Recyclable Hf-Based Polymeric Nanocatalyst. Polymers 2021, 13, 2808. https://doi.org/10.3390/polym13162808
Liu Y, Chen D, Li M, Zhang H, Li H. Catalytic Stereoselective Conversion of Biomass-Derived 4′-Methoxypropiophenone to Trans-Anethole with a Bifunctional and Recyclable Hf-Based Polymeric Nanocatalyst. Polymers. 2021; 13(16):2808. https://doi.org/10.3390/polym13162808
Chicago/Turabian StyleLiu, Yixuan, Dandan Chen, Mingrui Li, Heng Zhang, and Hu Li. 2021. "Catalytic Stereoselective Conversion of Biomass-Derived 4′-Methoxypropiophenone to Trans-Anethole with a Bifunctional and Recyclable Hf-Based Polymeric Nanocatalyst" Polymers 13, no. 16: 2808. https://doi.org/10.3390/polym13162808
APA StyleLiu, Y., Chen, D., Li, M., Zhang, H., & Li, H. (2021). Catalytic Stereoselective Conversion of Biomass-Derived 4′-Methoxypropiophenone to Trans-Anethole with a Bifunctional and Recyclable Hf-Based Polymeric Nanocatalyst. Polymers, 13(16), 2808. https://doi.org/10.3390/polym13162808