Characterization of Sodium Alginate Hydrogels Reinforced with Nanoparticles of Hydroxyapatite for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Synthesis of Hydrogels
2.2. Hydrogel Composite Characterization: Thermogravimetric Analysis
2.3. Differential Scanning Calorimetry (DSC)
2.4. Nuclear Magnetic Resonance
2.5. Mechanical Testing
2.6. X-ray Microtomography (Micro-CT)
- −
- 0% HA: source to object distance of 11.5 mm, to attain magnification of 27.8, with a pixel size of approximately 2.7 micrometers.
- −
- 20%: source to object distance of 10.7 mm, to attain magnification of 30.0, with a pixel size of approximately 2.5 micrometers.
- −
- 40%: source to object distance of 9.7 mm, to attain magnification of 33.1, with a pixel size of approximately 2.3 micrometers.
- −
- 60%: source to object distance of 11.3 mm, to attain magnification of 28.4, with a pixel size of approximately 2.6 micrometers.
Isolation and Quantification of Particles of HA
3. Results and Discussion
3.1. Thermogravimetric Analysis (TGA)
3.2. Differential Scanning Calorimetry (DSC)
3.3. Nuclear Magnetic Resonance (NMR)
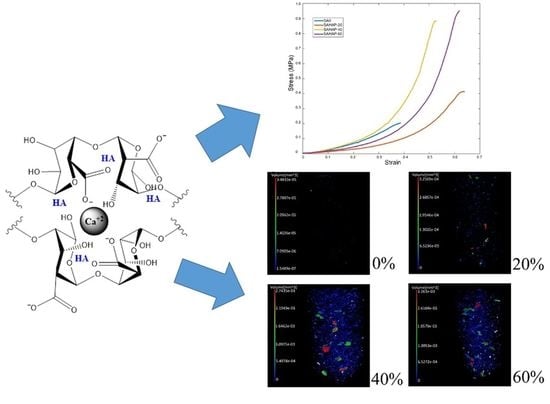
3.4. Mechanical Behavior
3.5. X-ray Microtomography (Micro-CT)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeegers, W.S.; Bohnen, L.M.L.J.; Laaper, M.; Verhaegen, M.J.A. Artificial disc replacement with the modular type SB Charité III: 2-year results in 50 prospectively studied patients. Eur. Spine J. 1999, 8, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Goda, E.S.; Gab-Allah, M.; Singu, B.S.; Yoon, K.R. Halloysite nanotubes based electrochemical sensors: A review. Microchem. J. 2019, 147, 1083–1096. [Google Scholar] [CrossRef]
- Hyde, P.; Tipper, J.; Fisher, J.; Hall, R. Wear and biological effects of a semi-constrained total disc replacement subject to modified ISO standard test conditions. J. Mech. Behav. Biomed. Mater. 2015, 44, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, C.; Mahabir, S.; Mohammed, K.; John, N.; Lee, K.-Y.; Ward, K. Calcium Alginate Thin Films Derived from Sargassum natans for the Selective Adsorption of Cd2+, Cu2+, and Pb2+ Ions. Ind. Eng. Chem. Res. 2019, 58, 1417–1425. [Google Scholar] [CrossRef]
- Chiew, C.S.C.; Poh, P.E.; Pasbakhsh, P.; Tey, B.T.; Yeoh, H.K.; Chan, E.S. Applied Clay Science Physicochemical characterization of halloysite / alginate bionanocomposite hydrogel. Appl. Clay Sci. 2014, 101, 444–454. [Google Scholar] [CrossRef]
- Murguía-Flores, D.A.; Bonilla-Ríos, J.; Canales-Fiscal, M.R.; Sánchez-Fernández, A. Protein adsorption through Chitosan–Alginate membranes for potential applications. Chem. Cent. J. 2016, 10, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Gullbrand, S.; Schaer, T.P.; Agarwal, P.; Bendigo, J.R.; Dodge, G.R.; Chen, W.; Elliott, D.M.; Mauck, R.L.; Malhotra, N.R.; Smith, L.J. Translation of an injectable triple-interpenetrating-network hydrogel for intervertebral disc regeneration in a goat model. Acta Biomater. 2017, 60, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Liu, M.; Long, Z.; Shen, Y.; Zhou, C. Effects of halloysite nanotubes on physical properties and cytocompatibility of alginate composite hydrogels. Mater. Sci. Eng. C 2017, 70, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-Y.; Zhu, Y.-J.; Li, H.; Zhang, Y.-G.; Shen, Y.-Q.; Sun, T.-W.; Chen, F. Preparation and enhanced mechanical properties of hybrid hydrogels comprising ultralong hydroxyapatite nanowires and sodium alginate. J. Colloid Interface Sci. 2017, 497, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Toti, U.S.; Aminabhavi, T.M. Different viscosity grade sodium alginate and modified sodium alginate membranes in pervapo-ration separation of water + acetic acid and water + isopropanol mixtures. J. Memb. Sci. 2004, 228, 199–208. [Google Scholar] [CrossRef]
- Davis, T.A.; Llanes, F.; Volesky, B.; Diaz-Pulido, G.; McCook, L.; Mucci, A. 1H-NMR Study of Na Alginates Extracted from Sargassum spp. in Relation to Metal Biosorption. Appl. Biochem. Biotechnol. 2003, 110, 75–90. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Lazzara, G.; Merli, M.; Milioto, S.; Parisi, F.; Sciascia, L. Effect of the Biopolymer Charge and the Nanoclay Morphology on Nanocomposite Materials. Ind. Eng. Chem. Res. 2016, 55, 7373–7380. [Google Scholar] [CrossRef]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Russo, R.; Malinconico, M.; Santagata, G. Effect of Cross-Linking with Calcium Ions on the Physical Properties of Alginate Films. Biomacromolecules 2007, 8, 3193–3197. [Google Scholar] [CrossRef]
- Li, L.; Fang, Y.; Vreeker, R.; Appelqvist, I.; Mendes, E. Reexamining the egg-box model in calcium–Alginate gels with X-ray dif-fraction. Biomacromolecules 2007, 8, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Behzadi, S. Performance comparison of two herbal and industrial medicines using nanoparticles with a starch/cellulose shell and alginate core for drug delivery: In vitro studies. Colloids Surf. B Biointerfaces 2017, 158, 556–561. [Google Scholar] [CrossRef]
- Sabbagh, N.; Akbari, A.; Arsalani, N.; Eftekhari-Sis, B.; Hamishekar, H. Halloysite-based hybrid bionanocomposite hydrogels as potential drug delivery systems. Appl. Clay Sci. 2017, 148, 48–55. [Google Scholar] [CrossRef]
- Sandri, G.; Aguzzi, C.; Rossi, S.; Bonferoni, M.C.; Bruni, G.; Boselli, C.; Cornaglia, A.I.; Riva, F.; Viseras, C.; Caramella, C.; et al. Halloysite and chitosan oligosaccharide nanocomposite for wound healing. Acta Biomater. 2017, 57, 216–224. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E. Fiji: An open-source platform for biological-image analysis. Nature methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 1–26. [Google Scholar] [CrossRef]
- Brun, F.; Mancini, L.; Kasae, P.; Favretto, S.; Dreossi, D.; Tromba, G. Pore3D: A software library for quantitative analysis of porous media. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2010, 615, 326–332. [Google Scholar] [CrossRef]
- Brus, J.; Urbanova, M.; Czernek, J.; Pavelkova, M.; Kubova, K.; Vyslouzil, J.; Abbrent, S.; Konefal, R.; Horský, J.; Vetchy, D.; et al. Structure and Dynamics of Alginate Gels Cross-Linked by Polyvalent Ions Probed via Solid State NMR Spectroscopy. Biomacromolecules 2017, 18, 2478–2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Nokab, M.E.H.; van der Wel, P.C. Use of solid-state NMR spectroscopy for investigating polysaccharide-based hydrogels: A review. Carbohydr. Polym. 2020, 240, 116276. [Google Scholar] [CrossRef]
- Huamani-Palomino, R.; Córdova, B.M.; Pichilingue, L.E.R.; Venâncio, T.; Valderrama, A. Functionalization of an Alginate-Based Material by Oxidation and Reductive Amination. Polymers 2021, 13, 255. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.; Jäger, H.; Ahmadi, S.J.; Lacroix, M. Electron beam crosslinking of alginate/nanoclay ink to improve functional properties of 3D printed hydrogel for removing heavy metal ions. Carbohydr. Polym. 2020, 240, 116211. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Barrias, C.C.; Almeida, I.F.; Costa, P.C.; Pena Ferreira, M.R.; Bahia, M.F.; Barbosa, M.A. Injectability of a Bone Filler System Based on Hydroxyapatite Microspheres and a Vehicle With in situ Gel-Forming Ability. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87, 49–58. [Google Scholar] [CrossRef]
- Douglas, T.E.L.; Schietse, J.; Zima, A.; Gorodzha, S.; Parakhonskiy, B.V.; KhaleNkow, D.; Shkarin, R.; Ivanova, A.; Baumbach, T.; Weinhardt, V.; et al. Novel self-gelling injectable hydrogel/alpha-TCP composites for bone regeneration: Physiochemical and micro-computer tomographical characterization. J. Biomed. Mater. Res. Part A. 2018, 106, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.R.; Canadas, R.F.; Silva-Correia, J.; da Silva Morais, A.; Oliveira, M.B.; Dias, I.R.; Mano, J.F.; Marques, A.P.; Reis, R.L.; Oliveira, J.M. Injectable gellan-gum/hydroxyapatite-based bilayered hydrogel composites forosteochondral tissue re-generation. Appl. Mater. Today 2018, 12, 309–321. [Google Scholar] [CrossRef] [Green Version]
- Lourenço, A.H.; Neves, N.; Ribeiro-Machado, C.; Sousa, S.R.; Lamghari, M.; Barrias, C.C.; Cabral, A.T.; Barbosa, M.A.; Ribeiro, C.C. Injectable hybrid system for strontium local delivery promotes bone regeneration in a rat critical-sized defect model. Sci. Rep. 2017, 7, 5098. [Google Scholar] [CrossRef] [Green Version]
- Moreau, D.; Villain, A.; Bachy, M.; Proudhon, H.; Ku, D.N.; Hannouche, D.; Petite, H.; Corté, L. In vivo evaluation of the bone integration of coated poly (vi-nyl-alcohol) hydrogel fiber implants. J. Mater. Sci. Mater. Med. 2017, 28, 114. [Google Scholar] [CrossRef]
- Liang, T.; Wu, J.; Li, F.; Huang, Z.; Pi, Y.; Miao, G.; Ren, W.; Liu, T.; Jiang, Q.; Guo, L. Drug-loading three-dimensional scaffolds based on hydroxyapatite-sodium alginate for bone regeneration. J. Biomed. Mater. Res. A 2021, 109, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zheng, X.; Liu, W.; Zhang, H.; Shao, J.; Yao, J.; Mao, C.; Hui, J.; Fan, D. A novel bovine serum albumin and sodium alginate hydrogel scaffold doped with hydroxyapatite nanowires for cartilage defects repair. Colloids Surfaces B Biointerfaces 2020, 192, 111041. [Google Scholar] [CrossRef] [PubMed]












| Sample | SA:NPs Ratio | CaCl2 |
|---|---|---|
| SA-0 | 1:0 | 5 wt.% |
| SAHA-20 | 4:1 | 5 wt.% |
| SAHA-40 | 3:2 | 5 wt.% |
| SAHA-60 | 2:3 | 5 wt.% |
| Sample | SAHA-20 (%) | SAHA-40 (%) | SAHA-60 (%) |
|---|---|---|---|
| Mass loss < 165 °C | 29.65 | 23.97 | 38.62 |
| Mass loss 165–230 °C | 5.23 | 3.90 | 3.90 |
| Mass loss 250–320 °C | 3.59 | 2.78 | 3.29 |
| Mass loss 350–500 °C | 2.29 | 2.57 | 4.66 |
| Signals (ppm) | SAHA-20 | SAHA-40 | SAHA-60 |
|---|---|---|---|
| 0.85 | 0.19 | 0.16 | 0.16 |
| 1.25 | 1.00 | 1.00 | 1.00 |
| 1.65 | 0.14 | 0.8 | 0.12 |
| 2.00 | 0.20 | 0.10 | 0.14 |
| 3.80 | 0.03 | 0.02 | 0.04 |
| Sample | UCS (MPa) | Increase (%) | E (MPa) | Increase (%) |
|---|---|---|---|---|
| SA-0 | 0.209 | - | 0.401 | - |
| SAHA-20 | 0.413 | 93.78 | 0.405 | 0.99 |
| SAHA-40 | 0.883 | 322.49 | 1.02 | 154.36 |
| SAHA-60 | 0.95 | 354.55 | 0.91 | 126.93 |
| Number of Particles | Volume of Particles [mm3] | Reconstructed Volume [mm3] | Volume of Particles per Reconstructed Volume [mm3/mm3] | Specific Surface Area (Sv): [mm−1] | |
|---|---|---|---|---|---|
| Hydrogel HA 0% | 397 | 3.34 × 10−4 | 7.92 | 4.22 × 10−5 | 0.03 |
| Hydrogel HA 20% | 1396 | 4.55 × 10−3 | 7.71 | 5.91 × 10−4 | 0.17 |
| Hydrogel HA 40% | 12,956 | 6.94 × 10−2 | 6.87 | 1.01 × 10−2 | 1.87 |
| Hydrogel HA 60% | 8127 | 7.11 × 10−2 | 5.73 | 1.24 × 10−2 | 1.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Fernández, J.A.; Presbítero-Espinosa, G.; Peña-Parás, L.; Pizaña, E.I.R.; Galván, K.P.V.; Vopálenský, M.; Kumpová, I.; Elizalde-Herrera, L.E. Characterization of Sodium Alginate Hydrogels Reinforced with Nanoparticles of Hydroxyapatite for Biomedical Applications. Polymers 2021, 13, 2927. https://doi.org/10.3390/polym13172927
Sánchez-Fernández JA, Presbítero-Espinosa G, Peña-Parás L, Pizaña EIR, Galván KPV, Vopálenský M, Kumpová I, Elizalde-Herrera LE. Characterization of Sodium Alginate Hydrogels Reinforced with Nanoparticles of Hydroxyapatite for Biomedical Applications. Polymers. 2021; 13(17):2927. https://doi.org/10.3390/polym13172927
Chicago/Turabian StyleSánchez-Fernández, José Antonio, Gerardo Presbítero-Espinosa, Laura Peña-Parás, Edgar Iván Rodríguez Pizaña, Katya Patricia Villarreal Galván, Michal Vopálenský, Ivana Kumpová, and Luis Ernesto Elizalde-Herrera. 2021. "Characterization of Sodium Alginate Hydrogels Reinforced with Nanoparticles of Hydroxyapatite for Biomedical Applications" Polymers 13, no. 17: 2927. https://doi.org/10.3390/polym13172927
APA StyleSánchez-Fernández, J. A., Presbítero-Espinosa, G., Peña-Parás, L., Pizaña, E. I. R., Galván, K. P. V., Vopálenský, M., Kumpová, I., & Elizalde-Herrera, L. E. (2021). Characterization of Sodium Alginate Hydrogels Reinforced with Nanoparticles of Hydroxyapatite for Biomedical Applications. Polymers, 13(17), 2927. https://doi.org/10.3390/polym13172927