1. Introduction
Ciprofibrate (CIP), chemical formula C
13H
14C
l2O
3, is classified as a synthetic active pharmaceutical ingredient (API), which belongs to the fibrate class of drugs, generally used against dyslipidemia, a condition characterized by abnormal lipid levels in the blood system [
1]. Dyslipidemia is a risk factor for developing cardiac diseases such as atherosclerosis, an inflammation characterized by the formation of fat, calcium, and other elements’ plates in the walls of the heart’s arteries and vascular system in general. Atherosclerosis can lead to different cardiac diseases responsible for more than 17.3 million deaths annually worldwide [
2]. Nowadays, CIP is commercially available for oral formulations as tablets and capsules (100 mg) [
3], and patients report several side effects such as headaches, nausea, and diarrhea [
4].
In pharmaceutical development, there is considerable interest in crystal structure investigations, as a way to understand how their structures (either amorphous or crystalline) and properties such as density, size, and particle shape correlate to other physicochemical properties, aiming the optimization of these drugs for solid dosage [
5].
A relevant characteristic of ciprofibrate is its hydrophobic behavior. Designated by the Biopharmaceutics Classification System (BCS) as a class II drug, CIP presents low solubility and high permeability [
6]. The high permeability allows a complete absorption of the drug by the small intestine; otherwise, its poor solubility limits its application to treatments. One possible way to overcome this problem is the use of micro- or nanostructures for improved drug delivery.
Nanomedicine—an interdisciplinary area that merges nanotechnology and medicine—has investigated several solutions that reach more efficient treatments with minimum side effects for various diseases. Several types of nanocarriers have been developed; structures such as polymeric micelles, liposomes, and NP that conjugate with drugs by diverse mechanisms (encapsulation, surface adsorption, and others) are used to deliver controlled and localized drug dosages to the body, implementing the drug delivery concept [
7,
8,
9,
10]. Each of these techniques presents a myriad of controllable features that can influence important aspects of the nanostructures. Encapsulation methods, in particular, may vary among different combinations of hydrophilic and lipophilic block copolymers and steps for synthesis, changing size, loading capacity, and even drug release behavior [
11,
12].
The poloxamer surfactant (or Pluronic, known by its trade name) is one of the most extensively investigated biomaterials used to build nanocarriers. These are typical triblock copolymers comprising two unities of poly (ethylene oxide) (PEO) and one of poly (propylene oxide) (PPO) in an alternated linear structure. Although not classified as biodegradable, PEO and derivatives present many advantages for biomedical usage such as relative safety profile (lethal dosage being LD50s > 5 g kg
−1), FDA approval, lack of immunogenicity, and particularly, the ease of excretion from living organisms [
13]. The remaining parts of the molecules are found in living systems since they are aliphatic chains (derived from fatty acid) and sugar molecules that are dietary sources of fuel and important structural components of cells. Additionally, taking into account the molecular weight of the amphiphilic chains (PCL Mw 18,500 g mol
−1, P123 Mw 5750 g mol
−1, and F127 12,600 g mol
−1), the materials can undergo renal clearance (Mw < 40,000 g mol
−1) [
13,
14]. Their block copolymer structures can self-assemble in different structural forms such as micelles, worm-like micelles, or vesicles in the aqueous medium. This approach allows the encapsulation of hydrophobic drugs such as CIP, increasing their solubility, improving circulation in vivo, and avoiding aggregation problems [
15].
Different types of Pluronic are used together as they present better properties such as colloidal stability and better drug-carrying efficiency [
15], especially for the self-assembly into micelle morphology [
16]. In this work, P123 and F127 block copolymers were used together (PEO
20-
b-PPO
70-
b-PEO
20 and PEO
100-
b-PPO
65-
b-PEO
100, respectively) to produce ciprofibrate-loaded micelles. F127 is a widely known and investigated copolymer for its thermoreversible gelation property at body temperature [
17]. Another block copolymer was considered for preparing the CIP-loaded micelles: the PEO
113-
b-PCL
118 was chosen due to its biocompatibility, biodegradability, and proven record in soft-based nanocarrier platforms for therapeutic applications [
18,
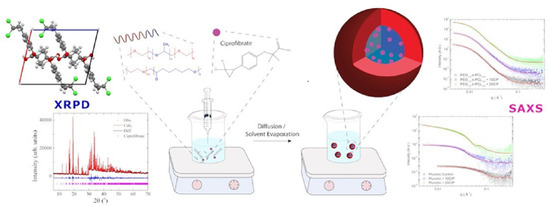
19]. The nanoprecipitation process was selected to prepare the CIP-loaded micelles, and an illustration of the method is provided in
Figure 1.
Recent results suggest that fenofibrate, which belongs to the same drug class as CIP, may have an essential role in controlling SARS-CoV-2 infection in in vitro models [
20]. Considering that the reduction in serum triglycerides and LDL cholesterol has a positive impact in the fighting against numerous age-related diseases and also against the recent COVID-19 infection, the relevance of more in-depth insight into ciprofibrate’s crystal structure to determine its fundamental properties and the possibility to use copolymer encapsulation as a strategy to improve its biodistribution becomes clear.
Herein, we present the crystal structure of CIP in solid state, solved using powder X-ray diffraction data. The drug was also encapsulated in both P123/F127 Pluronic and PEO113-b-PCL118 micelles for comparison via nanoprecipitation technique in water and evaluated in different solvent concentrations. We also propose the best condition for encapsulation for this system and demonstrate the fundamental physicochemical properties of the NPs (size distribution, surface charge, surface morphology). Furthermore, in vitro results and mathematical models for the drug release in buffer solution are presented.
2. Materials and Methods
CIP was obtained as a courtesy of DEINFAR (Laboratório de Desenvolvimento e Inovação Farmacotécnica) from the Faculty of Pharmaceutical Sciences (University of São Paulo, São Paulo, Brazil). PEO113-b-PCL118 blocks (Mw = 18,500 g mol−1) were purchased from Polymer Source, Inc. (Dorval, QC, Canada). Pluronic F127 (Mw = 12,600 g mol−1), Pluronic P123 (Mw = 5750 g mol−1), ethanol, acetone, PBS and Tween 20 were purchased from Sigma-Aldrich (São Paulo, Brazil).
2.1. Preparation of CIP-Loaded Nanoparticles
The polymeric micelles were produced by nanoprecipitation (as schematized in
Figure 1) from stock polymer/drug organic solutions prepared in ethanol. For Pluronic nanoparticles, first, P123 and F127 blocks were weighed and dissolved in ethanol (EtOH) (maintaining a 2:1
w/
w fixed molar ratio of P123 and F127). The polymer concentration was fixed at 10 mg mL
−1 as proposed by Sortini et al. [
21]. This solution was stabilized via sonication for approximately 10 min until it became visually transparent. The organic solution was transferred to a syringe and afterward added dropwise into 5 mL of water solution and then removed by evaporation. For the PEO
113-
b-PCL
118 nanoparticles, acetone was used to completely dissolve the polymeric chains [
6,
22]. For the CIP-loaded micelles, CIP was weighed and solubilized in ethanol according to the desired feeding and was mixed with the polymeric organic solution before the addition to the aqueous phase.
2.2. Particle Size and Morphology of CIP-Loaded Nanoparticles
The average diameter and size distribution (polydispersity) of the NPs were determined via Dynamic Light Scattering (DLS) and Static Light Scattering (SLS). Samples were loaded into test tubes (10 μL) and diluted in 1 mL of distilled water. Measurements were performed using ALV/CGS-3 platform-based goniometer system (ALV GmbH, Langen, Germany) consisting of a polarized HeNe laser (22 mW) operating at a wavelength λ = 633 nm, an ALV 7004 digital correlator, and a pair of pseudocorrelation APD detectors operating in a crusade mode. The data were collected and further averaged using ALV Correlator Control software. The polydispersity was estimated using the cumulant analysis of the autocorrelation functions measured at 90°. The temporal correlation functions were analyzed using the REPES algorithm (incorporated into the ALV Correlator program) to confirm the monomodal distribution of NPs. The autocorrelation functions reported are based on three independent runs of 60 s counting time for each sample.
The NPs’ surface charges were obtained via Electrophoretic Light Scattering (ELS) tests. Samples were added into cuvettes (10 µL), placed into the apparatus, and exposed to the laser beam. Experiments were carried out using a Zetasizer Nano-ZS ZEN3600 instrument (Malvern Instruments, Worcestershire, UK). The electrophoretic mobility (µe) was calculated through the Smoluchowski approximation. Each zeta-potential value reported is an average of 3 independent measurements with repeatability of ±2%.
2.3. X-ray Diffraction Analysis
CIP’s crystal structure was determined using Powder X-ray Diffraction (PXRD) data. Moreover, to the best of our knowledge, this is the first time it is reported. The method employed to solve the CIP’s crystal structure is well described in the literature [
6,
23]. The sample was hand-ground in an agate mortar and loaded between two cellulose acetate foils (0.014 mm) in a spinning sample holder. Powder X-ray diffraction data were collected utilizing a transmission mode copper source, filtered by a germanium monochromator (111). Diffraction intensities were collected by a linear detector Dectris Mythen 1K (Baden-Daettwil, Switzerland) with 0.015° step and integration time of 60 s at every 1.05°. The experiment used a STADI-P (Stoe, Darmstadt, Germany) powder diffractometer available at the Laboratory of Crystallography and Structural Characterization of Materials (LCCEM).
2.4. In Vitro Drug Release Characteristics of CIP-Loaded Nanoparticles
To measure the CIP release from the NPs and to compare the stabilities of both Pluronic and PEO113-b-PCL118, samples with 5 mg mL−1 containing 10% (w/w) and 20% (w/w) CIP were diluted in PBS pH 7.4 and placed in a dialysis bag (MWCO: 3.500–5.000 Da, Spectra/Por), which was dialyzed against 500 mL of PBS pH 7.4 containing 0.4% (w/v) Tween 20 at 37 °C for 48 h, under constant magnetic stirring. Aliquots of 50 µL were taken from the dialysis bag at increasing time intervals and afterward diluted 10 times in ethanol and measured through UV-Vis spectroscopy technique using a Cary 50 UV-Vis spectrophotometer (Varian, Inc., Crawley, UK). First, CIP’s analytical calibration curve in EtOH with a linear response in the range 0.0001–0.05 mg mL−1 was recorded and used to determine CIP contents. A sample containing empty NPs in EtOH was also measured as a blank sample for comparison. Then, samples were added into cuvettes (10 µL), diluted in EtOH, and placed into the equipment. Dilution proportions varied according to the different investigations, as some samples were highly concentrated and visually turbid, which may affect the results.
To describe the drug dissolution as a function of time, the drug release profile data were submitted to quantitative analysis, fitted to several kinetic release models. Statistical analysis was performed and indicated the models that best demonstrate the CIP’s release mechanism for both polymeric matrices.
2.5. Small-Angle X-ray Scattering
Small-Angle X-ray Scattering (SAXS) experiments were performed at the beamline B21 of the Diamond Light Source (Didcot, UK) [
23,
24]. Samples were loaded into quartz capillaries by the Arinax liquid-handling robot and exposed for 1 s, acquiring 20 frames. The wavelength was 0.95 Å, and the camera length was 3.71 m. Modeling to a spherical shell model was done using SASfit. It is worth noting that the samples were stored in a fridge at a range of 4–8 °C for three months before the SAXS experiment.
5. Conclusions
It was possible to characterize CIP’s crystal structure through this study, which has never been reported in the specific literature before. It crystallizes in a monoclinic crystal system in its solid-state, a pattern maintained due to strong H bonds between the –OH terminations of the molecules. We have also proposed a synthesis method and two polymeric matrices to encapsulate CIP and improve its solubility—a mixed proportion of Pluronic P123/F127 and a matrix composed of PEO
113-
b-PCL
118. These polymers are already well established as biopolymers but have not been tested yet with CIP mainly. Results indicate that both systems produced micelles with suitable physicochemical characteristics, such as small size and relatively neutral zeta potential (from −10 to +10 mV), with great potential to enhance delivery efficiency in the human body [
34]. The synthesis method was also demonstrated to be suitable, as repeated productions led to similar samples with minimum deviations. Besides, polydispersity values for the samples were very low, ideal for a stable solution.
As for the shape of the NPs, morphological characteristics demonstrated good agreement among diverse techniques such as DLS, SLS, SAXS, and statistical analysis using in vitro tests results after UV-Vis spectrophotometry for both systems. The SAXS profiles corroborate the spherical shape expected by combining the DLS/SLS measurements for both polymeric systems, except for the Pluronic micelles with 20 wt% of CIP loading, which presented a morphological change, as discussed adequately in
Section 4.3. The small values of the gyration radius also agree with the hydrodynamic radius. Moreover, comparing the scattering densities, results indicate that the drug was incorporated in the nucleus of the polymeric nanoparticles, as we expected.
In vitro release experiments also have indicated that the drug remains in the system for up to 48 h. Although both polymeric systems have shown very similar physicochemical characteristics, PEO
113-
b-PCL
118 micelles showed zero-order release kinetics in in vitro drug release tests. Statistical analyses also indicated that the proposed CIP-loaded mixed Pluronic system has a release mechanism based on Fickian diffusion, which is independent of the amount of CIP encapsulated. In conclusion, concerning the NPs’ physicochemical stability over time, this study proposes that CIP quantities up to 20% (
w/
w) can be encapsulated preferentially in PEO
113-
b-PCL
118 micelles as an alternative to increase its hydrophilicity and, therefore, its release time. Considering the recent advances in nanomedicine and the continued efforts to improve drug delivery techniques in order to provide targeted release of drugs, hydrophobicity, and bioavailability [
7,
35,
36], this study represents an initial step to understand both CIP’s physicochemical properties and the proposed nanoparticle system of encapsulation. The present work may also contribute to studies aiming to better comprehend the mechanism of action of the fibrate class drugs in diminishing lipid levels in the human body and, beyond that, to the rising number of studies correlating the presence of dyslipidemia condition with more severe forms of COVID-19 infection [
37,
38,
39]. Therefore, further studies can be conducted for possible improvements in ciprofibrate’s pharmaceutical applications.