Fabrication of Al2O3/ZnO and Al2O3/Cu Reinforced Silicone Rubber Composite Pads for Thermal Interface Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Rubbery Composite Pads
2.3. Characterization
3. Results and Discussion
3.1. Preparation of Al2O3 Reinforced Rubbery Composite Pads
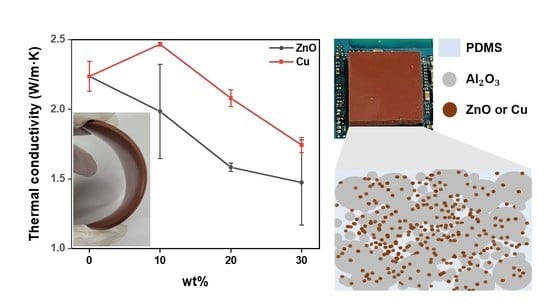
3.2. Preparation of Al2O3/ZnO Reinforced Rubbery Composite Pads
3.3. Preparation of Al2O3/Cu Reinforced Rubbery Composite Pads
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, W.; Yu, D.; Min, C.; Fu, Y.; Guo, X. Thermal, dielectric, and mechanical properties of SiC particles filled linear low-density polyethylene composites. J. Appl. Polym. Sci. 2009, 112, 1695–1703. [Google Scholar] [CrossRef]
- Prasher, R. Thermal Interface Materials: Historical Perspective, Status, and Future Directions. Proc. IEEE 2006, 94, 1571–1586. [Google Scholar] [CrossRef]
- Gwinn, J.; Webb, R. Performance and testing of thermal interface materials. Microelectron. J. 2003, 34, 215–222. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Ting, J.-M. Ultra high thermal conductivity polymer composites. Carbon 2002, 40, 359–362. [Google Scholar] [CrossRef]
- Lee, G.-W.; Park, M.; Kim, J.; Lee, J.I.; Yoon, H.G. Enhanced thermal conductivity of polymer composites filled with hybrid filler. Compos. Part A Appl. Sci. Manuf. 2006, 37, 727–734. [Google Scholar] [CrossRef]
- Song, Y.S.; Youn, J.R. Influence of dispersion states of carbon nanotubes on physical properties of epoxy nanocomposites. Carbon 2005, 43, 1378–1385. [Google Scholar] [CrossRef]
- Zhou, W.-Y.; Qi, S.-H.; Zhao, H.-Z.; Liu, N.-L. Thermally conductive silicone rubber reinforced with boron nitride particle. Polym. Compos. 2007, 28, 23–28. [Google Scholar] [CrossRef]
- Kalaprasad, G.; Pradeep, P.; Mathew, G.; Pavithran, C.; Thomas, S. Thermal conductivity and thermal diffusivity analyses of low-density polyethylene composites reinforced with sisal, glass and intimately mixed sisal/glass fibres. Compos. Sci. Technol. 2000, 60, 2967–2977. [Google Scholar] [CrossRef]
- Yang, K.; Gu, M. Enhanced thermal conductivity of epoxy nanocomposites filled with hybrid filler system of triethylenetetramine-functionalized multi-walled carbon nanotube/silane-modified nano-sized silicon carbide. Compos. Part A Appl. Sci. Manuf. 2010, 41, 215–221. [Google Scholar] [CrossRef]
- Kumari, L.; Zhang, T.; Du, G.H.; Li, W.Z.; Wang, Q.W.; Datye, A.; Wu, K.H. Thermal properties of CNT-Alumina nanocomposites. Compos. Sci. Technol. 2008, 68, 2178–2183. [Google Scholar] [CrossRef]
- Uetani, K.; Ata, S.; Tomonoh, S.; Yamada, T.; Yumura, M.; Hata, K. Elastomeric Thermal Interface Materials with High Through-Plane Thermal Conductivity from Carbon Fiber Fillers Vertically Aligned by Electrostatic Flocking. Adv. Mater. 2014, 26, 5857–5862. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Qi, S.; Li, H.; Shao, S. Study on insulating thermal conductive BN/HDPE composites. Thermochim. Acta 2006, 452, 36–42. [Google Scholar] [CrossRef]
- Hsieh, C.-Y.; Chung, S.-L. High thermal conductivity epoxy molding compound filled with a combustion synthesized AlN powder. J. Appl. Polym. Sci. 2006, 102, 4734–4740. [Google Scholar] [CrossRef]
- Si, W.; He, X.; Huang, Y.; Gao, X.; Zheng, X.; Zheng, X.; Leng, C.; Su, F.; Wuyan, S. Polydimethylsiloxane/aluminum oxide composites prepared by spatial confining forced network assembly for heat conduction and dissipation. RSC Adv. 2018, 8, 36007–36014. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Hu, J.; Zhang, C.; Wang, J.; Chen, L.; Bian, X.; Lin, J.; Du, X. Performance improvement in nano-alumina filled silicone rubber composites by using vinyl tri-methoxysilane. Polym. Test. 2018, 67, 295–301. [Google Scholar] [CrossRef]
- Nielsen, L.E. Thermal conductivity of particulate-filled polymers. J. Appl. Polym. Sci. 1973, 17, 3819–3820. [Google Scholar] [CrossRef]
- Dowbenko, R.; Hart, D.P. Nonaqueous Dispersions as Vehicles for Polymer Coatings. Ind. Eng. Chem. Prod. Res. Dev. 1973, 12, 14–28. [Google Scholar] [CrossRef]
- Mamunya, Y.; Davydenko, V.; Pissis, P.; Lebedev, E. Electrical and thermal conductivity of polymers filled with metal powders. Eur. Polym. J. 2002, 38, 1887–1897. [Google Scholar] [CrossRef]
- Pal, R. On the Lewis–Nielsen model for thermal/electrical conductivity of composites. Compos. Part A Appl. Sci. Manuf. 2008, 39, 718–726. [Google Scholar] [CrossRef]
- Cebeci, H.; de Villoria, R.G.; Hart, A.J.; Wardle, B.L. Multifunctional properties of high volume fraction aligned carbon nanotube polymer composites with controlled morphology. Compos. Sci. Technol. 2009, 69, 2649–2656. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, X.; Zhu, Y.; Hui, D.; Qiu, Y. Mechanical, electrical and thermal properties of aligned carbon nanotube/polyimide composites. Compos. Part B Eng. 2013, 56, 408–412. [Google Scholar] [CrossRef]
- Kumlutaş, D.; Tavman, İ.H.; Turhan Çoban, M. Thermal conductivity of particle filled polyethylene composite materials. Compos. Sci. Technol. 2003, 63, 113–117. [Google Scholar]
- Sim, L.; Ramanan, S.; Ismail, H.; Seetharamu, K.; Goh, T. Thermal characterization of Al2O3 and ZnO reinforced silicone rubber as thermal pads for heat dissipation purposes. Thermochim. Acta 2005, 430, 155–165. [Google Scholar] [CrossRef]
- Slack, G.A. Nonmetallic crystals with high thermal conductivity. J. Phys. Chem. Solids 1973, 34, 321–335. [Google Scholar] [CrossRef]
- Slack, G.A. Thermal conductivity of MgO, Al2O3, MgAl2O4, and Fe3O4, crystals from 3° to 300 °K. Phys. Rev. 1962, 126, 427–441. [Google Scholar] [CrossRef]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with designer functionalities—Properties, modifications strategies, and applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Tanaka, T.; Kozako, M.; Okamoto, K. Toward High Thermal Conductivity Nano Micro Epoxy Composites with Sufficient Endurance Voltage. J. Int. Counc. Electr. Eng. 2012, 2, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Kim, J. Thermal conductivity of epoxy composites with a binary-particle system of aluminum oxide and aluminum nitride fillers. Compos. Part B Eng. 2013, 51, 140–147. [Google Scholar] [CrossRef]
- Kwon, Y.-J.; Park, J.-B.; Jeon, Y.-P.; Hong, J.-Y.; Park, H.-S.; Lee, J.-U. A Review of Polymer Composites Based on Carbon Fillers for Thermal Management Applications: Design, Preparation, and Properties. Polymers 2021, 13, 1312. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, S.; Long, Y.; Zhu, P.; Wu, F.; Liu, F.; Murugadoss, V.; Winchester, W.; Nautiyal, A.; Wang, Z.; et al. Recent advances in thermal interface materials. ES Mater. Manuf. 2020, 7, 4–24. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Fang, Z.; Huang, Y.; Xu, H.; Liu, Y.; Wu, D.; Zhuang, J.; Sun, J. Recent Advances in Preparation, Mechanisms, and Applications of Thermally Conductive Polymer Composites: A Review. J. Compos. Sci. 2020, 4, 180. [Google Scholar] [CrossRef]
- Santiago-Alvarado, A.; Cruz-Felix, A.; Iturbide, F.; Licona-Morán, B. Physical-chemical properties of PDMS samples used in tunable lenses. Int. J. Eng. Sci. Innov. Technol. 2014, 3, 563–571. [Google Scholar]
- Gao, B.; Xu, J.; Peng, J.; Kang, F.; Du, H.; Li, J.; Chiang, S.; Xu, C.; Hu, N.; Ning, X. Experimental and theoretical studies of effective thermal conductivity of composites made of silicone rubber and Al2O3 particles. Thermochim. Acta 2015, 614, 1–8. [Google Scholar] [CrossRef]
- Mu, Q.; Feng, S.; Diao, G. Thermal conductivity of silicone rubber filled with ZnO. Polym. Compos. 2007, 28, 125–130. [Google Scholar] [CrossRef]
- Vincent, C.; Silvain, J.-F.; Heintz, J.-M.; Chandra, N. Effect of porosity on the thermal conductivity of copper processed by powder metallurgy. J. Phys. Chem. Solids 2012, 73, 499–504. [Google Scholar] [CrossRef]
- Yi, P.; Awang, R.A.; Rowe, W.; Kalantar-Zadeh, K.; Khoshmanesh, K. PDMS nanocomposites for heat transfer enhancement in microfluidic platforms. Lab Chip 2014, 14, 3419–3426. [Google Scholar] [CrossRef] [PubMed]










| PDMS | Al2O3 Powder | ZnO Powder | Cu Powder | ||
|---|---|---|---|---|---|
| Size (μm) | - | 70 | 5 | 1 | 1 |
| Density (g/cm3) | 0.982 | 3.87 | 3.74 | 5.47 | 8.9 |
| Thermal conductivity (W/m∙K) | 0.27 | 30 | 60 | 400 | |
| Type | Matrix (PDMS) | Filler | ||||||
|---|---|---|---|---|---|---|---|---|
| Base | Curing Agent | 70 μm Al2O3 | 5 μm Al2O3 | |||||
| wt% | vol% | wt% | vol% | wt% | vol% | wt% | vol% | |
| A | 19 | 46.73 | 1 | 2.458 | 40 | 24.97 | 40 | 25.84 |
| B | 19 | 46.84 | 1 | 2.464 | 50 | 31.27 | 30 | 19.42 |
| C | 19 | 46.95 | 1 | 2.470 | 60 | 37.61 | 20 | 12.98 |
| D | 14.25 | 38.16 | 0.75 | 2.045 | 45 | 31.14 | 40 | 28.65 |
| E | 14.25 | 38.69 | 0.75 | 2.036 | 55 | 37.89 | 30 | 21.39 |
| F | 14.25 | 38.77 | 0.75 | 2.041 | 65 | 44.89 | 20 | 14.29 |
| Type | Matrix (PDMS) | Filler | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Base | Curing Agent | 70 μm Al2O3 | 5 μm Al2O3 | 1 μm ZnO | ||||||
| wt% | vol% | wt% | vol% | wt% | vol% | wt% | vol% | wt% | vol% | |
| B | 19 | 46.84 | 1 | 2.464 | 50 | 31.28 | 30 | 19.42 | 0 | 0 |
| B-ZnO 10 | 19 | 47.82 | 1 | 2.516 | 50 | 31.93 | 20 | 13.22 | 10 | 4.518 |
| B-ZnO 20 | 19 | 48.84 | 1 | 2.570 | 50 | 32.61 | 10 | 6.749 | 20 | 9.228 |
| B-ZnO 30 | 19 | 49.91 | 1 | 2.626 | 50 | 33.32 | 0 | 0 | 30 | 14.14 |
| E | 14.25 | 38.69 | 0.75 | 2.036 | 55 | 37.89 | 30 | 21.39 | 0 | 0 |
| E-ZnO 10 | 14.25 | 39.59 | 0.75 | 2.084 | 55 | 38.77 | 20 | 14.59 | 10 | 4.988 |
| E-ZnO 20 | 14.25 | 40.52 | 0.75 | 2.132 | 55 | 39.68 | 10 | 7.466 | 20 | 10.21 |
| E-ZnO 30 | 14.25 | 41.50 | 0.75 | 2.184 | 55 | 40.64 | 0 | 0 | 30 | 15.68 |
| Type | Matrix (PDMS) | Filler | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Base | Curing Agent | 70 μm Al2O3 | 5 μm Al2O3 | 1 μm Cu | ||||||
| wt% | vol% | wt% | vol% | wt% | vol% | wt% | vol% | wt% | vol% | |
| B | 19 | 46.84 | 1 | 2.464 | 50 | 31.28 | 30 | 19.42 | 0 | 0 |
| B-Cu 10 | 19 | 48.67 | 1 | 2.560 | 50 | 32.49 | 20 | 13.45 | 10 | 2.827 |
| B-Cu 20 | 19 | 50.64 | 1 | 2.664 | 50 | 33.81 | 10 | 6.998 | 20 | 5.881 |
| B-Cu 30 | 19 | 52.78 | 1 | 2.777 | 50 | 35.24 | 0 | 0 | 30 | 9.196 |
| E | 14.25 | 38.69 | 0.75 | 2.036 | 55 | 37.89 | 30 | 21.39 | 0 | 0 |
| E-Cu 10 | 14.25 | 40.36 | 0.75 | 2.124 | 55 | 39.52 | 20 | 14.87 | 10 | 3.126 |
| E-Cu 20 | 14.25 | 42.17 | 0.75 | 2.220 | 55 | 41.30 | 10 | 7.772 | 20 | 6.531 |
| E-Cu 30 | 14.25 | 44.16 | 0.75 | 2.324 | 55 | 43.25 | 0 | 0 | 30 | 10.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.; Choi, E.J.; Cheon, H.J.; Choi, W.I.; Shin, W.S.; Lim, J.-M. Fabrication of Al2O3/ZnO and Al2O3/Cu Reinforced Silicone Rubber Composite Pads for Thermal Interface Materials. Polymers 2021, 13, 3259. https://doi.org/10.3390/polym13193259
Jang S, Choi EJ, Cheon HJ, Choi WI, Shin WS, Lim J-M. Fabrication of Al2O3/ZnO and Al2O3/Cu Reinforced Silicone Rubber Composite Pads for Thermal Interface Materials. Polymers. 2021; 13(19):3259. https://doi.org/10.3390/polym13193259
Chicago/Turabian StyleJang, Seokkyu, Eun Ji Choi, Han Jin Cheon, Won Il Choi, Woon Seo Shin, and Jong-Min Lim. 2021. "Fabrication of Al2O3/ZnO and Al2O3/Cu Reinforced Silicone Rubber Composite Pads for Thermal Interface Materials" Polymers 13, no. 19: 3259. https://doi.org/10.3390/polym13193259
APA StyleJang, S., Choi, E. J., Cheon, H. J., Choi, W. I., Shin, W. S., & Lim, J. -M. (2021). Fabrication of Al2O3/ZnO and Al2O3/Cu Reinforced Silicone Rubber Composite Pads for Thermal Interface Materials. Polymers, 13(19), 3259. https://doi.org/10.3390/polym13193259