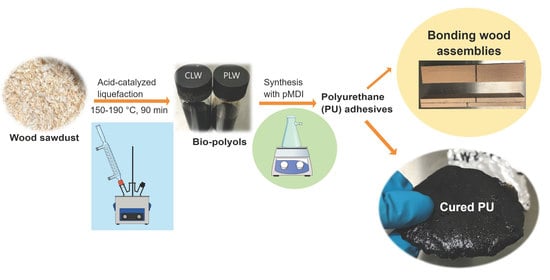
Preparation of Polyurethane Adhesives from Crude and Purified Liquefied Wood Sawdust
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Liquefaction Process
2.3. Characterization of Polyols
2.4. Preparation of PU Adhesive
2.5. Bonding Strength of PU Adhesives
2.6. Adhesive Penetration
2.7. Analytical Characterization of Polyols and Cured PU Adhesives
2.8. Statistical Analysis
3. Results and Discussion
3.1. Liquefaction Yield and Polyol Characteristics
3.2. Performance of PU Adhesives
3.3. Analytical Characterization of Cured PU Adhesives
3.3.1. FTIR Spectra
3.3.2. TGA
4. Conclusions and Outlook for Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lay, D.G.; Cranley, P. Polyurethane adhesives. In Handbook of Adhesive Technology; CRC Press: Boca Raton, FL, USA, 2003; pp. 695–718. [Google Scholar]
- Sharmin, E.; Zafar, F. Polyurethane: An Introduction. In Polyurethane, 1st ed.; Zafar, F., Sharmin, E., Eds.; Intech: Rijeka, Croatia, 2012; pp. 3–6. [Google Scholar]
- Somarathna, H.; Raman, S.; Mohotti, D.; Mutalib, A.; Badri, K. The use of polyurethane for structural and infrastructural engineering applications: A state-of-the-art review. Constr. Build. Mater. 2018, 190, 995–1014. [Google Scholar] [CrossRef]
- Patel, M.R.; Shukla, J.M.; Patel, N.K.; Patel, K.H. Biomaterial based novel polyurethane adhesives for wood to wood and metal to metal bonding. Mater. Res. 2009, 12, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Dotan, A. Biobased thermosets. In Handbook of Thermoset Plastics, 3rd ed.; Dodiuk, H., Goodman, S.H., Eds.; William Andrew Publishing: Boston, MA, USA, 2014; pp. 577–622. [Google Scholar]
- Alinejad, M.; Henry, C.; Nikafshar, S.; Gondaliya, A.; Bagheri, S.; Chen, N.; Singh, S.K.; Hodge, D.B.; Nejad, M. Lignin-based polyurethanes: Opportunities for bio-based foams, elastomers, coatings and adhesives. Polymers 2019, 11, 1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawpan, M.A. Polyurethanes from vegetable oils and applications: A review. J. Polym. Res. 2018, 25, 184. [Google Scholar] [CrossRef]
- Sahoo, S.; Mohanty, S.; Nayak, S.K. Biobased polyurethane adhesive over petroleum based adhesive: Use of renewable resource. J. Macromol. Sci. Part A 2018, 55, 36–48. [Google Scholar] [CrossRef]
- Li, Y.; Luo, X.; Hu, S. Introduction to bio-based polyols and polyurethanes. In Bio-Based Polyols and Polyurethanes; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2015; pp. 1–13. [Google Scholar]
- Rajalingam, P.; Radhakrishnan, G. Poly (chloroprene)-castor oil based polyurethane semi-interpenetrating polymer network as an adhesive. Polym. Int. 1991, 25, 87–90. [Google Scholar] [CrossRef]
- Xi, X.; Wu, Z.; Pizzi, A.; Gerardin, C.; Lei, H.; Zhang, B.; Du, G. Non-isocyanate polyurethane adhesive from sucrose used for particleboard. Wood Sci. Technol. 2019, 53, 393–405. [Google Scholar] [CrossRef]
- Chen, X.; Pizzi, A.; Essawy, H.; Fredon, E.; Gerardin, C.; Guigo, N.; Sbirrazzuoli, N. Non-furanic humins-based non-isocyanate polyurethane (NIPU) thermoset wood adhesives. Polymers 2021, 13, 372. [Google Scholar] [CrossRef]
- Borrero-López, A.M.; Guzmán, D.B.; González-Delgado, J.A.; Arteaga, J.F.; Valencia, C.; Pischel, U.; Franco, J.M. Toward UV-triggered curing of solvent-free polyurethane adhesives based on castor oil. ACS Sustain. Chem. Eng. 2021, 9, 11032–11040. [Google Scholar] [CrossRef]
- Cornille, A.; Michaud, G.; Simon, F.; Fouquay, S.; Auvergne, R.; Boutevin, B.; Caillol, S. Promising mechanical and adhesive properties of isocyanate-free poly (hydroxyurethane). Eur. Polym. J. 2016, 84, 404–420. [Google Scholar] [CrossRef]
- Figovsky, O.L.; Shapovalov, L.D. Nonisocyanate polyurethanes for adhesives and coatings. In Proceedings of the First International IEEE Conference on Polymers and Adhesives in Microelectronics and Photonics, Potsdam, Germany, 21–24 October 2001. [Google Scholar]
- Dolci, E.; Michaud, G.; Simon, F.; Fouquay, S.; Boutevin, B.; Caillol, S. Remendable thermosetting polymers for isocyanate-free adhesives: A preliminary study. Polym. Chem. 2015, 6, 7851–7861. [Google Scholar] [CrossRef]
- Somani, K.P.; Kansara, S.S.; Patel, N.K.; Rakshit, A.K. Castor oil based polyurethane adhesives for wood-to-wood bonding. Int. J. Adhes. Adhes. 2003, 23, 269–275. [Google Scholar] [CrossRef]
- Sahoo, S.; Kalita, H.; Mohanty, S.; Nayak, S.K. Synthesis and characterization of vegetable oil based polyurethane derived from low viscous bio aliphatic isocyanate: Adhesion strength to wood-wood substrate bonding. Macromol. Res. 2017, 25, 772–778. [Google Scholar] [CrossRef]
- Moghadam, P.N.; Yarmohamadi, M.; Hasanzadeh, R.; Nuri, S. Preparation of polyurethane wood adhesives by polyols formulated with polyester polyols based on castor oil. Int. J. Adhes. Adhes. 2016, 68, 273–282. [Google Scholar] [CrossRef]
- Malik, M.; Kaur, R. Mechanical and thermal properties of castor oil-based polyurethane adhesive: Effect of TiO2 Filler. Adv. Polym. Technol. 2018, 37, 24–30. [Google Scholar] [CrossRef]
- Dias, F.M.; Lahr, F.A.R. Alternative castor oil-based polyurethane adhesive used in the production of plywood. Mater. Res. 2004, 7, 413–420. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Tai, W. Castor oil-based polyurethane resin for low-density composites with bamboo charcoal. Polymers 2018, 10, 1100. [Google Scholar] [CrossRef] [Green Version]
- Su, Q.; Wei, D.; Dai, W.; Zhang, Y.; Xia, Z. Designing a castor oil-based polyurethane as bioadhesive. Colloids Surf. B Biointerfaces 2019, 181, 740–748. [Google Scholar] [CrossRef]
- Huang, J.; Li, A.; Li, K. Investigation of polyurethane-based pressure-sensitive adhesives with castor oil as a polyol. Int. J. Adhes. Adhes. 2020, 105, 102763. [Google Scholar] [CrossRef]
- Desai, S.D.; Patel, J.V.; Sinha, V.K. Polyurethane adhesive system from biomaterial-based polyol for bonding wood. Int. J. Adhes. Adhes. 2003, 23, 393–399. [Google Scholar] [CrossRef]
- Mishra, D.; Sinha, V.K. Eco-economical polyurethane wood adhesives from cellulosic waste: Synthesis, characterization and adhesion study. Int. J. Adhes. Adhes. 2010, 30, 47–54. [Google Scholar] [CrossRef]
- Gosz, K.; Kowalkowska-Zedler, D.; Haponiuk, J.; Piszczyk, Ł. Liquefaction of alder wood as the source of renewable and sustainable polyols for preparation of polyurethane resins. Wood Sci. Technol. 2020, 54, 103–121. [Google Scholar] [CrossRef]
- Lee, W.-J.; Lin, M.-S. Preparation and application of polyurethane adhesives made from polyhydric alcohol liquefied Taiwan acacia and China fir. J. Appl. Polym. Sci. 2008, 109, 23–31. [Google Scholar] [CrossRef]
- Lee, W.-J.; Chao, C.-Y. Effect of containing polyhydric alcohol liquefied wood on the properties of thermoplastic polyurethane resins. Eur. J. Wood Wood Prod. 2018, 76, 1745–1752. [Google Scholar] [CrossRef]
- Juhaida, M.; Paridah, M.; Hilmi, M.M.; Sarani, Z.; Jalaluddin, H.; Zaki, A.M. Liquefaction of kenaf (Hibiscus cannabinus L.) core for wood laminating adhesive. Bioresour. Technol. 2010, 101, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Sankar, G.; Yan, N. Bio-based two component (2 K) polyurethane adhesive derived from liquefied infested lodgepole pine barks. J. Biobased Mater. Bioenergy 2014, 8, 457–464. [Google Scholar] [CrossRef]
- Kong, X.; Liu, G.; Curtis, J.M. Characterization of canola oil based polyurethane wood adhesives. Int. J. Adhes. Adhes. 2011, 31, 559–564. [Google Scholar] [CrossRef]
- Nacas, A.; Ito, N.M.; De Sousa, R.R.; Spinacé, M.A.; dos Santos, D. Effects of NCO:OH ratio on the mechanical properties and chemical structure of Kraft lignin-based polyurethane adhesive. J. Adhes. 2017, 93, 18–29. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Y.; Fu, X.; Kong, W.; Liu, Z.; Hu, K.; Jiang, L.; Lei, J. Molecular design for silane-terminated polyurethane applied to moisture-curable pressure-sensitive adhesive. J. Appl. Polym. Sci. 2017, 134, 45292. [Google Scholar] [CrossRef]
- Malucelli, G.; Priola, A.; Ferrero, F.; Quaglia, A.; Frigione, M.; Carfagna, C. Polyurethane resin-based adhesives: Curing reaction and properties of cured systems. Int. J. Adhes. Adhes. 2005, 25, 87–91. [Google Scholar] [CrossRef]
- Lu, Y.; LaRock, R. Aqueous cationic polyurethane dispersions from vegetable oils. ChemSusChem 2010, 3, 329–333. [Google Scholar] [CrossRef]
- Cui, S.; Luo, X.; Li, Y. Synthesis and properties of polyurethane wood adhesives derived from crude glycerol-based polyols. Int. J. Adhes. Adhes. 2017, 79, 67–72. [Google Scholar] [CrossRef]
- Yuan, L.; Qiang, P.; Gao, J.; Shi, Y. Synthesis of oxazolidines as latent curing agents for single-component polyurethane adhesive and its properties study. J. Appl. Polym. Sci. 2018, 135, 45722. [Google Scholar] [CrossRef]
- Dodangeh, F.; Dorraji, M.S.; Rasoulifard, M.; Ashjari, H. Synthesis and characterization of alkoxy silane modified polyurethane wood adhesive based on epoxidized soybean oil polyester polyol. Compos. Part B Eng. 2020, 187, 107857. [Google Scholar] [CrossRef]
- Jiang, W.; Kumar, A.; Adamopoulos, S. Liquefaction of lignocellulosic materials and its applications in wood adhesives—A review. Ind. Crop. Prod. 2018, 124, 325–342. [Google Scholar] [CrossRef]
- Hassan, E.B.; Shukry, N. Polyhydric alcohol liquefaction of some lignocellulosic agricultural residues. Ind. Crop. Prod. 2008, 27, 33–38. [Google Scholar] [CrossRef]
- Briones, R.; Serrano, L.; Llano-Ponte, R.; Labidi, J. Polyols obtained from solvolysis liquefaction of biodiesel production solid residues. Chem. Eng. J. 2011, 175, 169–175. [Google Scholar] [CrossRef]
- D’Souza, J.; Camargo, R.; Yan, N. Biomass liquefaction and alkoxylation: A review of structural characterization methods for bio-based polyols. Polym. Rev. 2017, 57, 668–694. [Google Scholar] [CrossRef]
- Hu, S.; Luo, X.; Li, Y. Polyols and polyurethanes from the liquefaction of lignocellulosic biomass. ChemSusChem 2013, 7, 66–72. [Google Scholar] [CrossRef]
- Kunaver, M.; Jasiukaitytė, E.; Čuk, N.; Guthrie, J.T. Liquefaction of wood, synthesis and characterization of liquefied wood polyester derivatives. J. Appl. Polym. Sci. 2009, 115, 1265–1271. [Google Scholar] [CrossRef]
- Daneshvar, S.; Behrooz, R.; Kazemi Najafi, S.; Mir Mohamad Sadeghi, G. Characterization of polyurethane wood adhesive prepared from liquefied sawdust by ethylene carbonate. BioResouces 2018, 14, 20. [Google Scholar]
- Ionescu, M. Chemistry and Technology of Polyols for Polyurethanes; iSmithers Rapra Publishing: Shrewsbury, UK, 2005. [Google Scholar]
- Zhou, W.; Fu, W.; Zhang, Y. Liquefaction of banana pseudo-stem and preparation of polyurethane adhesive from liquefied products. J. Wuhan Univ. Technol. Sci. Ed. 2018, 33, 1437–1443. [Google Scholar] [CrossRef]
- ASTM. Astm d4274: Standard Test Methods for Testing Polyurethane Raw Materials-Determination of Hydroxyl Numbers of Polyols; ASTM International Danvers: West Conshohocken, PA, USA, 1999. [Google Scholar]
- ASTM. Astm D2572-97: Standard Test Method for Isocyanate Groups in Urethane Materials or Prepolymers; ASTM International: West Conshohocken, PA, USA, 1997. [Google Scholar]
- European Standard. EN 302-1: Adhesives for Load-Bearing Timber Structures—Test Methods—Part 1: Determination of Bond Strength in Longitudinal Tensile Shear Strength; European Committee for Standardization: Brussels, Belgium, 2013. [Google Scholar]
- Bastani, A.; Adamopoulos, S.; Militz, H. Gross adhesive penetration in furfurylated, N-methylol melamine-modified and heat-treated wood examined by fluorescence microscopy. Eur. J. Wood Wood Prod. 2015, 73, 635–642. [Google Scholar] [CrossRef]
- Sernek, M.; Resnik, J.; Kamke, F.A. Penetration of liquid urea-formaldehyde adhesive into beech wood. Wood Fiber Sci. 2007, 31, 41–48. [Google Scholar]
- Zhang, H.; Ding, F.; Luo, C.; Xiong, L.; Chen, X. Liquefaction and characterization of acid hydrolysis residue of corncob in polyhydric alcohols. Ind. Crop. Prod. 2012, 39, 47–51. [Google Scholar] [CrossRef]
- Braz, A.; Mateus, M.M.; dos Santos, R.G.; Machado, R.; Bordado, J.M.; Correia, M.J.N. Modelling of pine wood sawdust thermochemical liquefaction. Biomass Bioenergy 2019, 120, 200–210. [Google Scholar] [CrossRef]
- D’Souza, J.; Wong, S.Z.; Camargo, R.; Yan, N. Solvolytic liquefaction of bark: Understanding the role of polyhydric alcohols and organic solvents on polyol characteristics. ACS Sustain. Chem. Eng. 2016, 4, 851–861. [Google Scholar] [CrossRef]
- Xie, T.; Chen, F. Fast liquefaction of bagasse in ethylene carbonate and preparation of epoxy resin from the liquefied product. J. Appl. Polym. Sci. 2005, 98, 1961–1968. [Google Scholar] [CrossRef]
- Kurimoto, Y.; Koizumi, A.; Doi, S.; Tamura, Y.; Ono, H. Wood species effects on the characteristics of liquefied wood and the properties of polyurethane films prepared from the liquefied wood. Biomass Bioenergy 2001, 21, 381–390. [Google Scholar] [CrossRef]
- Lee, S.-H.; Teramoto, Y.; Shiraishi, N. Biodegradable polyurethane foam from liquefied waste paper and its thermal stability, biodegradability, and genotoxicity. J. Appl. Polym. Sci. 2002, 83, 1482–1489. [Google Scholar] [CrossRef]
- Zheng, Z.; Pan, H.; Huang, Y.; Chung, Y.; Zhang, X.; Feng, H. Rapid liquefaction of wood in polyhydric alcohols under microwave heating and its liquefied products for preparation of rigid polyurethane foam. Open Mater. Sci. J. 2011, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ugovšek, A.; Sernek, M. Effect of pressing parameters on the shear strength of beech specimens bonded with low solvent liquefied wood. J. Adhes. Sci. Technol. 2013, 27, 182–195. [Google Scholar] [CrossRef]
- Serrano, L.; Rincón, E.; García, A.; Rodríguez, J.; Briones, R. Bio-degradable polyurethane foams produced by liquefied polyol from wheat straw biomass. Polymers 2020, 12, 2646. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Jelle, B.; Hovde, P.; Rüther, P. Ftir spectroscopy as a tool to predict service life of wooden cladding. In CIB World Congress; Centre Scientifique et Technique du Bâtiment: Paris, France, 2010; pp. 10–13. [Google Scholar]
- Fackler, K.; Stevanic, J.S.; Ters, T.; Hinterstoisser, B.; Schwanninger, M.; Salmén, L. FT-IR imaging microscopy to localise and characterise simultaneous and selective white-rot decay within spruce wood cells. Holzforschung 2011, 65, 411–420. [Google Scholar] [CrossRef]
- Nejad, S.M.M.; Madhoushi, M.; Vakili, M.; Rasouli, D. Evaluation of degradation in chemical compounds of wood in historical buildings using FT-IR and FT-Raman vibrational spectroscopy. Maderas. Ciencia Tecnologia 2019, 21, 381–392. [Google Scholar] [CrossRef] [Green Version]
- Lionetto, F.; Del Sole, R.; Cannoletta, D.; Vasapollo, G.; Maffezzoli, A. Monitoring wood degradation during weathering by cellulose crystallinity. Materials 2012, 5, 1910–1922. [Google Scholar] [CrossRef] [Green Version]
- Cuello, C.; Marchand, P.; Laurans, F.; Grand-Perret, C.; Lainé-Prade, V.; Pilate, G.; Déjardin, A. ATR-FTIR Microspectroscopy Brings a Novel Insight into the Study of Cell Wall Chemistry at the Cellular Level. Front. Plant Sci. 2020, 11, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traoré, M.; Kaal, J.; Cortizas, A.M. Differentiation between pine woods according to species and growing location using FTIR-ATR. Wood Sci. Technol. 2018, 52, 487–504. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Xing, D.; Lia, J. FTIR studies of the changes in wood chemistry from wood forming tissue under inclined treatment. Energy Procedia 2012, 16, 758–762. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.; De, A.; De, U. Structural and optical investigations of radiation damage in transparent PET polymer films. Int. J. Spectrosc. 2011, 2011, 810936. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, S.; Egüés, I.; Labidi, J. Liquefaction of kraft lignin using polyhydric alcohols and organic acids as catalysts for sustainable polyols production. Ind. Crop. Prod. 2019, 137, 687–693. [Google Scholar] [CrossRef]
- Kobayashi, M.; Asano, T.; Kajiyama, M.; Tomita, B. Analysis on residue formation during wood liquefaction with polyhydric alcohol. J. Wood Sci. 2004, 50, 407–414. [Google Scholar] [CrossRef]
- Yamada, T.; Ono, H. Characterization of the products resulting from ethylene glycol liquefaction of cellulose. J. Wood Sci. 2001, 47, 458–464. [Google Scholar] [CrossRef]
- D’Souza, J.; Yan, N. Producing bark-based polyols through liquefaction: Effect of liquefaction temperature. ACS Sustain. Chem. Eng. 2013, 1, 534–540. [Google Scholar] [CrossRef]
- Kosmela, P.; Hejna, A.; Formela, K.; Haponiuk, J.; Piszczyk, Ł. Biopolyols obtained via. crude glycerol-based liquefaction of cellulose: Their structural, rheological and thermal characterization. Cellulose 2016, 23, 2929–2942. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, R.G.; Carvalho, R.; Silva, E.R.; Bordado, J.; Cardoso, A.C.; Costa, M.D.R.; Mateus, M. Natural polymeric water-based adhesive from cork liquefaction. Ind. Crop. Prod. 2016, 84, 314–319. [Google Scholar] [CrossRef]
- Briones, R.; Rodriguez, J.; Labidi, J.; Cunningham, E.; Martin, P. Liquefaction of corn husks and properties of biodegradable biopolyol blends. J. Chem. Technol. Biotechnol. 2020, 95, 2973–2982. [Google Scholar] [CrossRef]
- Kariz, M.; Sernek, M. Bonding of heat-treated spruce with phenol-formaldehyde adhesive. J. Adhes. Sci. Technol. 2010, 24, 1703–1716. [Google Scholar] [CrossRef]
- Hemmilä, V.; Adamopoulos, S.; Hosseinpourpia, R.; Ahmed, S.A. Ammonium lignosulfonate adhesives for particleboards with pMDI and furfuryl alcohol as crosslinkers. Polymers 2019, 11, 1633. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.S.; Badri, K.H. Chemical analyses of palm kernel oil-based polyurethane prepolymer. Mater. Sci. Appl. 2012, 3, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Webster, D.C. New biobased high functionality polyols and their use in polyurethane coatings. ChemSusChem 2012, 5, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, M.F. Introduction to polyurehtane chemistry. In Polyurethanes: Science, Technology, Markets, and Trends; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 105–124. [Google Scholar]
- Sonnenschein, M.F. Polyurethanes: Science, Technology, Markets, and Trends; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Javni, I.; Petrović, Z.S.; Guo, A.; Fuller, R. Thermal stability of polyurethanes based on vegetable oils. J. Appl. Polym. Sci. 2000, 77, 1723–1734. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Kairytė, A. Application of walnut shells-derived biopolyol in the synthesis of rigid polyurethane foams. Materials 2020, 13, 2687. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Gao, L.; Guo, W. Effect of incorporation of lignin as bio-polyol on the performance of rigid lightweight wood-polyurethane composite foams. J. Wood Sci. 2020, 66, 23. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.-Y.; De Hoop, C.F.; Peng, X.-P.; Xie, J.-L.; Qi, J.-Q.; Jiang, Y.-Z.; Xiao, H.; Nie, S.-X. Thermal stability analysis of polyurethane foams made from microwave liquefaction bio-polyols with and without solid residue. Bioresources 2018, 13, 3346–3361. [Google Scholar] [CrossRef]









| Year | Developing Strategies | Remarks | Reference |
|---|---|---|---|
| 1991 | Poly(chloroprene)-castor oil based polyurethane adhesives | Good adhesion for rubber to rubber bonding | [10] |
| 2001–2021 | Non-isocyanate polyurethane adhesives | Need of high curing temperature; renewability; non-toxicity | [11,12,13,14,15,16] |
| 2003–2021 | Castor oil based polyurethane adhesives for wood bonding | Highly crosslinked polymeric structure; low cost; renewablility | [17,18,19,20,21,22,23,24] |
| 2003 | Polyurethane adhesives based on polyester polyols from potato starch and natural oils | Good bonding property; highly cross-linked structure; good water resistance | [25] |
| 2005 | Polyurethane wood adhesives based on palm kernel oil | Good adhesion property; low cost | [26] |
| 2008–2020 | Polyurethane adhesives based on bio-polyols from liquefaction of lignocellulosic materials | Abundant availability of raw materials; rich in hydroxyl groups; stable aromatic polymer structure | [27,28,29,30,31] |
| 2011 | Polyurethane wood adhesives based on canola oil | Low cost; good bonding property and chemical resistance; superior hot water resistance | [32] |
| 2016 | Polyurethane adhesive based on kraft lignin as a polyol | High reactivity of the hydroxyl groups in kraft lignin; use of industrial waste | [33] |
| 2017 | Moisture curable silane-terminated polyurethane adhesives | Avoidance of CO2 production | [34,35,36] |
| 2017 | Polyurethane wood adhesives from crude glycerol-based polyols | Use of the waste stream of biodiesel production; low cost; renewablility | [37] |
| 2018 | Application of oxazolidine compounds in one-component polyurethane adhesives | Decreased bubble number and size; better bondability | [38] |
| 2020 | Polyurethane wood adhesives based on polyester polyol from soybean oil | Improved performance by addition of additives | [39] |
| 2021 | Solvent-free polyurethane adhesives based on castor oil and organic diisocyanates | Fast curing; low adhesion | [13] |
| NCO:OH Molar Ratio | CLWPU | PLWPU |
|---|---|---|
| 0.5:1 | CLWPU1 | PLWPU1 |
| 1:1 | CLWPU2 | PLWPU2 |
| 1.5:1 | CLWPU3 | PLWPU3 |
| 2:1 | CLWPU4 | PLWPU4 |
| Properties | CLW | PLW |
|---|---|---|
| OH number (mg KOH/g) | 825 ± 11 | 623 ± 8 |
| Acid number (mg·KOH/g) | 48.2 ± 1.1 | 47.8 ± 1.4 |
| Viscosity (mPa·s at 20 °C) | 3900 | 3700 |
| pH | 0.19 | 0.22 |
| Material | Tonset (°C) | T5wt.% (°C) | T10wt.% (°C) | Tmax1 (°C) | Tmax2 (°C) | Toffset (°C) |
|---|---|---|---|---|---|---|
| CLW | 50 | 117 | 137 | 189 | 396 | 489 |
| PLW | 50 | 109 | 134 | 200 | 391 | 499 |
| Material | Tonset (°C) | T5wt.% (°C) | T10wt.% (°C) | Tmax1 (°C) | Tmax2 (°C) | Tmax3 (°C) | Toffset (°C) |
|---|---|---|---|---|---|---|---|
| CLWPU1 | 111 | 131 | 161 | 202 | 380 | - | 637 |
| CLWPU2 | 100 | 179 | 241 | 285 | 358 | - | 614 |
| CLWPU3 | 190 | 203 | 275 | - | 361 | 460 | 630 |
| CLWPU4 | 227 | 224 | 295 | - | 363 | 487 | 630 |
| PLWPU1 | 110 | 165 | 178 | 265 | 360 | - | 615 |
| PLWPU2 | 114 | 169 | 230 | - | 355 | - | 608 |
| PLWPU3 | 167 | 202 | 271 | - | 356 | - | 617 |
| PLWPU4 | 215 | 224 | 274 | - | 359 | 460 | 618 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Hosseinpourpia, R.; Biziks, V.; Ahmed, S.A.; Militz, H.; Adamopoulos, S. Preparation of Polyurethane Adhesives from Crude and Purified Liquefied Wood Sawdust. Polymers 2021, 13, 3267. https://doi.org/10.3390/polym13193267
Jiang W, Hosseinpourpia R, Biziks V, Ahmed SA, Militz H, Adamopoulos S. Preparation of Polyurethane Adhesives from Crude and Purified Liquefied Wood Sawdust. Polymers. 2021; 13(19):3267. https://doi.org/10.3390/polym13193267
Chicago/Turabian StyleJiang, Wen, Reza Hosseinpourpia, Vladimirs Biziks, Sheikh Ali Ahmed, Holger Militz, and Stergios Adamopoulos. 2021. "Preparation of Polyurethane Adhesives from Crude and Purified Liquefied Wood Sawdust" Polymers 13, no. 19: 3267. https://doi.org/10.3390/polym13193267
APA StyleJiang, W., Hosseinpourpia, R., Biziks, V., Ahmed, S. A., Militz, H., & Adamopoulos, S. (2021). Preparation of Polyurethane Adhesives from Crude and Purified Liquefied Wood Sawdust. Polymers, 13(19), 3267. https://doi.org/10.3390/polym13193267