Glycolysis of Polyurethanes Composites Containing Nanosilica
Abstract
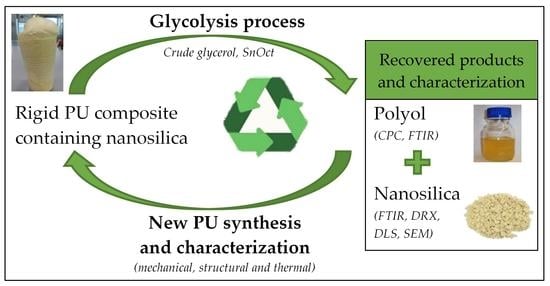
:1. Introduction
2. Experimental
2.1. Materials
2.1.1. Glycolysis
2.1.2. Polyurethane Synthesis
2.2. Glycolysis Process
2.3. Polyurethane Synthesis Process
2.4. Characterization Techniques
2.4.1. Molecular Weight and Product Composition Determination by Gel Permeation Chromatography (GPC)
2.4.2. Structural Studies Using Fourier Transform Infrared Spectroscopy (FTIR)
2.4.3. Measurement of Hydroxyl Index (iOH)
2.5. Nanosilica Shape and Size Characterization
2.5.1. Dynamic Light Scattering (DLS)
2.5.2. Scanning Electron Microscopy (SEM)
2.6. Foams Characterization
2.6.1. Apparent Density
2.6.2. Compression Test
2.6.3. Foam Cell Structure (SEM)
2.6.4. Effective Conductivity
2.7. Distillation Process
3. Results and Discussion
3.1. Feasibility Study of the Glycolysis Process of Rigid PU Foams with Nanosilica as a Filler
3.1.1. Characterization of Upper and Bottom Phases
3.1.2. Characterization of Solid Phase
3.2. Synthesis of Rigid PU Foams Using Recovered Polyol and Filler
3.3. Characterization of the Synthesized Rigid PU Foams: Influence of the Recovered Polyol and Filler
3.3.1. Apparent Density
3.3.2. Compression Test
3.3.3. PU Foams Thermal Characterization
3.3.4. PU Foams Structural Characterization
3.4. Recovery of the Glycolysis Agent
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hicks, D.; Austin, A. A Review of the Global PU Industry in 2016 and Outlook for 2017; Dr. Gupta Verlag: Ratingen, Germany, 2017; Volume 14. [Google Scholar]
- Kiss, G.; Rusu, G.; Peter, F.; Tănase, I.; Bandur, G. Recovery of Flexible Polyurethane Foam Waste for Efficient Reuse in Industrial Formulations. Polymers 2020, 12, 1533. [Google Scholar] [CrossRef]
- Kanchanapiya, P.; Intaranon, N.; Tantisattayakul, T. Assessment of the economic recycling potential of a glycolysis treatment of rigid polyurethane foam waste: A case study from Thailand. J. Environ. Manag. 2021, 280, 111638. [Google Scholar] [CrossRef]
- Peyrton, J.; Avérous, L. Structure-properties relationships of cellular materials from biobased polyurethane foams. Mater. Sci. Eng. R Rep. 2021, 145, 100608. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Gutiérrez, C.; Rodríguez, J.F. Sustainable polyurethanes: Chemical recycling to get it. In Handbook of Environmental Chemistry; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Das, A.; Mahanwar, P. A brief discussion on advances in polyurethane applications. Adv. Ind. Eng. Polym. Res. 2020. [Google Scholar] [CrossRef]
- Bengtström, L.; Salden, M.; Stec, A.A. The role of isocyanates in fire toxicity. Fire Sci. Rev. 2016, 5, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Trzebiatowska, P.J.; Echart, A.S.; Correas, T.C.; Eceiza, A.; Datta, J. The changes of crosslink density of polyurethanes synthesised with using recycled component. Chemical structure and mechanical properties investigations. Prog. Org. Coat. 2018, 115, 41–48. [Google Scholar] [CrossRef]
- Yang, W.; Dong, Q.; Liu, S.; Xie, H.; Liu, L.; Li, J. Recycling and Disposal Methods for Polyurethane Foam Wastes. Procedia Environ. Sci. 2012, 16, 167–175. [Google Scholar] [CrossRef]
- Nikje, M.M.A.; Tehrani, Z.M. Novel modified nanosilica-based on synthesized dipodal silane and its effects on the physical properties of rigid polyurethane foams. Des. Monomers Polym. 2010, 13, 249–260. [Google Scholar] [CrossRef]
- Santiago-Calvo, M.; Tirado-Mediavilla, J.; Ruiz-Herrero, J.L.; Rodríguez-Pérez, M.Á.; Villafañe, F. The effects of functional nanofillers on the reaction kinetics, microstructure, thermal and mechanical properties of water blown rigid polyurethane foams. Polymer (Guildf.) 2018, 150, 138–149. [Google Scholar] [CrossRef]
- Benli, S.; Yilmazer, Ü.; Pekel, F.; Özkar, S. Effect of fillers on thermal and mechanical properties of polyurethane elastomer. J. Appl. Polym. Sci. 1998, 68, 1057–1065. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Strzelec, K.; Kairytė, A.; Vaitkus, S. Composites of rigid polyurethane foams and silica powder filler enhanced with ionic liquid. Polym. Test. 2019, 75, 12–25. [Google Scholar] [CrossRef]
- Do, T.V.V.; Le, V.H.V.; Thai, N.U.N.; Dai, H.N.; Grillet, A.-C.; Thuc, C.N.H. The influence of nano-silica on the thermal conductivity of polyurethane foam. J. Appl. Polym. Sci. 2021, 50715. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Rodríguez, J.F. Recycling of polyurethanes from laboratory to industry, a journey towards the sustainability. Waste Manag. 2018, 76, 147–171. [Google Scholar] [CrossRef]
- Heiran, R.; Ghaderian, A.; Reghunadhan, A.; Sedaghati, F.; Thomas, S.; Haghighi, A.H. Glycolysis: An efficient route for recycling of end of life polyurethane foams. J. Polym. Res. 2021, 28, 22. [Google Scholar] [CrossRef]
- AOCS. AOCS Official Method. In Official Methods and Recommended Practices of the American Oils Chemists Society; Springer: Cham, Switzerland, 2009. [Google Scholar]
- Borreguero, M.; Sánchez, M.L.; Valverde, J.L.; Carmona, M.; Rodríguez, J.F. Thermal testing and numerical simulation of gypsum wallboards incorporated with different PCMs content. Appl. Energy 2011, 88, 930–937. [Google Scholar] [CrossRef]
- Serrano; Borreguero, A.M.; Garrido, I.; Rodríguez, J.F.; Carmona, M. Reducing heat loss through the building envelope by using polyurethane foams containing thermoregulating microcapsules. Appl. Therm. Eng. 2016, 103, 226–232. [Google Scholar] [CrossRef]
- Simón, D.; García, M.T.; de Lucas, A.; Borreguero, A.M.; Rodríguez, J.F. Glycolysis of flexible polyurethane wastes using stannous octoate as the catalyst: Study on the influence of reaction parameters. Polym. Degrad. Stab. 2013, 98, 144–149. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Rodríguez, J.F. Glycolysis of flexible polyurethane wastes containing polymeric polyols. Polym. Degrad. Stab. 2014, 109, 115–121. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Rodríguez, J.F. Glycolysis of viscoelastic flexible polyurethane foam wastes. Polym. Degrad. Stab. 2015, 116, 23–35. [Google Scholar] [CrossRef]
- Simón, D.; de Lucas, A.; Rodríguez, J.F.; Borreguero, A.M. Glycolysis of high resilience flexible polyurethane foams containing polyurethane dispersion polyol. Polym. Degrad. Stab. 2016, 133, 119–130. [Google Scholar] [CrossRef]
- Bhabhe, M.D.; Athawale, V.D. Gel permeation chromatographic method for monitoring the transesterification reaction in a two-step chemoenzymatic synthesis of urethane oil based on vegetable oils. J. Chromatogr. A 1995, 718, 299–304. [Google Scholar] [CrossRef]
- Libretexst, Infrared Spectroscopy Absorption Table. Sonoma State Univ. Saves Students Hard Cash with Libr. 2013. Available online: https://chem.libretexts.org/Ancillary_Materials/Reference/Reference_Tables/Spectroscopic_Parameters/Infrared_Spectroscopy_Absorption_Table (accessed on 1 January 2021).
- Trzebiatowska, P.J.; Beneš, H.; Datta, J. Evaluation of the glycerolysis process and valorisation of recovered polyol in polyurethane synthesis. React. Funct. Polym. 2019, 133, 119–130. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Rodríguez, J.F. Valorization of crude glycerol as a novel transesterification agent in the glycolysis of polyurethane foam waste. Polym. Degrad. Stab. 2015, 121, 126–136. [Google Scholar] [CrossRef]
- Simón, D.; de Lucas, A.; Rodríguez, J.F.; Borreguero, A.M. Flexible polyurethane foams synthesized employing recovered polyols from glycolysis: Physical and structural properties. J. Appl. Polym. Sci. 2017, 134, 45087. [Google Scholar] [CrossRef]
- Tran, T.N.; Pham, T.V.A.; Le, M.L.P.; Nguyen, T.P.T.; Tran, V.M. Synthesis of amorphous silica and sulfonic acid functionalized silica used as reinforced phase for polymer electrolyte membrane. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 045007. [Google Scholar] [CrossRef] [Green Version]
- Mukharjee, B.; Barai, S.V. Influence of Nano-Silica on the properties of recycled aggregate concrete. Constr. Build. Mater. 2014, 55, 29–37. [Google Scholar] [CrossRef]
- Campos, H.; Oportus, M.; Torres, C.; Urbina, C.; Fierro, J.L.G.; Reyes, P. Enantioselective hydrogenation of 1-phenyl-propane-1,2-dione on immobilised cinchonidine Pt/SiO2 catalysts. J. Mol. Catal. A Chem. 2011, 348, 30–41. [Google Scholar] [CrossRef]
- Sendijarevicy, V. Chemical recycling of mixed polyurethane foam stream recovered from shredder residue into polyurethane polyols. J. Cell. Plast. 2007, 43, 31–46. [Google Scholar] [CrossRef]
- De Lucas, A.M.; Romero, F.; Rodriguez, J.F. Influence of the use of recycled polyols obtained by glycolysis on the preparation and physical properties of flexible polyurethane. J. Appl. Polym. Sci. 2008, 109, 617–626. [Google Scholar]
- Simón, D.; Rodríguez, J.F.; Carmona, M.; Serrano, A.; Borreguero, A.M. Glycolysis of advanced polyurethanes composites containing thermoregulating microcapsules. Chem. Eng. J. 2018, 350, 300–311. [Google Scholar] [CrossRef]
- Borreguero, M.; Rodríguez, J.F.; Valverde, J.L.; Arevalo, R.; Peijs, T.; Carmona, M. Characterization of rigid polyurethane foams containing microencapsulated Rubitherm® RT27: Catalyst effect. Part II. J. Mater. Sci. 2011, 46, 347–356. [Google Scholar] [CrossRef]
- Borreguero, M.; Valverde, J.L.; Peijs, T.; Rodríguez, J.F.; Carmona, M. Characterization of rigid polyurethane foams containing microencapsulated Rubitherm® RT27. Part I. J. Mater. Sci. 2010, 45, 4462–4469. [Google Scholar] [CrossRef]
- Ghasemi, S.; Amini, E.N.; Tajvidi, M.; Kiziltas, A.; Mielewski, D.F.; Gardner, D.J. Flexible polyurethane foams reinforced with organic and inorganic nanofillers. J. Appl. Polym. Sci. 2021, 138, 49983. [Google Scholar] [CrossRef]
- Lan, Z.; Daga, R.; Whitehouse, R.; McCarthy, S.; Schmidt, D. Structure-properties relations in flexible polyurethane foams containing a novel bio-based crosslinker. Polymer (Guildf.) 2014, 55, 2635–2644. [Google Scholar] [CrossRef]
- Giebeler, R.G.; Krause, H.; Fisch, R.; Musso, F.; Petzinka, K.H. Refurbishment Manual: Maintenance, Conversions, Extensions; Walter de Gruyter: Berlin, Germany, 2009. [Google Scholar]
- Griffin, J.; Brotzen, F.R.; Loos, P.J. Effect of thickness on the transverse thermal conductivity of thin dielectric films. J. Appl. Phys. 1994, 75, 3761–3764. [Google Scholar] [CrossRef]
- Kraitape, N.; Thongpin, C. Influence of recycled polyurethane polyol on the properties of flexible polyurethane foams. Energy Proc. 2016, 89, 186–197. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Fernández, S.; Günther, M.; Schartel, B.; Corcuera, M.A.; Eceiza, A. Impact of the combined use of layered double hydroxides, lignin and phosphorous polyol on the fire behavior of flexible polyurethane foams. Ind. Crops Prod. 2018, 125, 346–359. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Molero, C.; Rodríguez, J.F. Novel polyol initiator from polyurethane recycling residue. J. Mater. Cycles Waste Manag. 2014, 16, 525–532. [Google Scholar] [CrossRef]
















| PU Foam:DEG. | 1:1.5 | 1:2 |
|---|---|---|
| Reagent | Mass (g) | |
| PU foam | 50 | 50 |
| Glycolysis Agent | 73.3 | 97.7 |
| Tin Octoate | 1.7 | 2.3 |
| Upper Phaset % | Bottom Phase wt % | Molecular Weight (g/mol) | |
|---|---|---|---|
| Recovered polyol (peaks I, II, III and IV) | 76.5 | 8.5 | 638 (714, 643, 558, 423) |
| Reaction by-products | - | 19.4 | 360 |
| Crude glycerol | 23.5 | 72.1 | 92 |
| Product | Mn (g/mol) | OHnumber (mg KOH/g) | Functionality |
|---|---|---|---|
| Commercial rigid polyether polyols | 300–700 | 250–1000 | 3–8 |
| Upper phase (recovered polyol) | 509 | 854 | 7.75 |
| P100 R0 | P85 R15 | P70 R30 | P55 R45 | P40 R60 | P25 R75 | |
|---|---|---|---|---|---|---|
| Raw rigid polyether polyol OH = 455 mg KOH/g | 100 | 85 | 70 | 55 | 40 | 25 |
| Recovered polyol OH = 853.73 mg KOH/g | 0 | 15 | 30 | 45 | 60 | 75 |
| Polyol mixture hydroxyl number (mg KOH/g) | 455 | 514.81 | 574.62 | 634.43 | 694.24 | 754.05 |
| Tegoamin BDE | 2.5 | |||||
| Water | 2.5 | |||||
| Tegostab B8404 | 1.5 | |||||
| Nanosilica (recovered or fresh) | 6.18 | 6.54 | 6.90 | 7.25 | 7.61 | 7.97 |
| PMDI | 157.13 | 172.35 | 187.56 | 202.78 | 217.99 | 233.21 |
| PU Foam Sample. | Apparent Density, ρ (Kg/m3) | Standard Deviation | |
|---|---|---|---|
| Nanosilica | Recovered Polyol (wt %) | ||
| Without nanosilica | 0.0 | 45.61 | ±0.40 |
| Fresh nanosilica | 0.0 | 49.65 | ±0.29 |
| Recovered nanosilica | 0.0 | 48.15 | ±0.49 |
| 15.0 | 58.52 | ±0.21 | |
| 30.0 | 63.15 | ±0.45 | |
| 45.0 | 64.05 | ±0.33 | |
| 60.0 | 64.97 | ±0.31 | |
| Recovered Polyol (wt %) | Viscosity, μ (Pa·s) | Standard Deviation |
|---|---|---|
| 0.0 | 3.85 | ±0.11 |
| 15.0 | 6.81 | ±0.09 |
| 30.0 | 11.11 | ±0.47 |
| 45.0 | 13.49 | ±0.56 |
| 60.0 | 15.68 | ±0.12 |
| 75.0 | 17.61 | ±0.08 |
| PU Foam Sample | Maximum Compressive Strength σ (kPa) | Standard Deviation | Young’s Modulus E (MPa) | Standard Deviation | |
|---|---|---|---|---|---|
| Nanosilica | Recovered Polyol (wt %) | ||||
| Without nanosilica | 0.0 | 469.78 | ±4.70 | 11.60 | ±0.08 |
| Fresh nanosilica | 0.0 | 537.01 | ±2.68 | 13.61 | ±0.14 |
| Recovered nanosilica | 0.0 | 510.34 | ±1.96 | 13.50 | ±0.05 |
| 15.0 | 514.72 | ±7.94 | 13.30 | ±0.13 | |
| 30.0 | 522.62 | ±5.65 | 12.76 | ±0.15 | |
| 45.0 | 540.62 | ±4.61 | 10.37 | ±0.04 | |
| 60.0 | 552.01 | ±5.37 | 9.47 | ±0.06 | |
| PU Foam Sample | Effective Thermal Conductivity, k (W/m·K) | Standard Deviation | |
|---|---|---|---|
| Nanosilica | Recovered Polyol (wt %) | ||
| Without nanosilica | 0.0 | 0.041 | ±0.003 |
| Fresh nanosilica | 0.0 | 0.062 | ±0.002 |
| Recovered nanosilica | 0.0 | 0.064 | ±0.003 |
| 15.0 | 0.065 | ±0.003 | |
| 30.0 | 0.063 | ±0.004 | |
| 45.0 | 0.062 | ±0.001 | |
| 60.0 | 0.063 | ±0.001 | |
| CG employed in glycolysis process (g) | 73.3 |
| Recovered CG from BP (g) | 40.24 |
| Recovery yield of CG (%) | 54.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Amo, J.; Borreguero, A.M.; Ramos, M.J.; Rodríguez, J.F. Glycolysis of Polyurethanes Composites Containing Nanosilica. Polymers 2021, 13, 1418. https://doi.org/10.3390/polym13091418
del Amo J, Borreguero AM, Ramos MJ, Rodríguez JF. Glycolysis of Polyurethanes Composites Containing Nanosilica. Polymers. 2021; 13(9):1418. https://doi.org/10.3390/polym13091418
Chicago/Turabian Styledel Amo, Jesus, Ana Maria Borreguero, Maria Jesus Ramos, and Juan Francisco Rodríguez. 2021. "Glycolysis of Polyurethanes Composites Containing Nanosilica" Polymers 13, no. 9: 1418. https://doi.org/10.3390/polym13091418
APA Styledel Amo, J., Borreguero, A. M., Ramos, M. J., & Rodríguez, J. F. (2021). Glycolysis of Polyurethanes Composites Containing Nanosilica. Polymers, 13(9), 1418. https://doi.org/10.3390/polym13091418