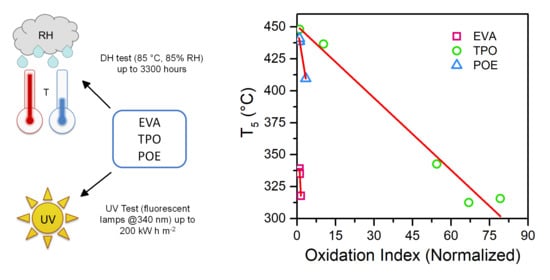
Comparison of Degradation Behavior of Newly Developed Encapsulation Materials for Photovoltaic Applications under Different Artificial Ageing Tests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thermal Desorption Gas Chromatography Coupled to Mass Spectrometry (TD-GC/MS)
- held for two minutes at 50 °C,
- from 50 C to 290 °C, held at 290 °C for six minutes, heating rate of 10 C min−1,
- ion source temperature and interface temperature were set at 300 °C,
- splitless mode.
2.2. UV-Visible-Near Infrared Spectroscopy (UV-Vis-NIR)
2.3. Fourier Transform Infrared Spectroscopy (FT-IR)
2.4. Differential Scanning Calorimetry (DSC)
2.5. Thermogravimetric Analysis (TGA)
3. Results and Discussion
3.1. Thermal Desorption Gas Chromatography Coupled to Mass Spectrometry (TD-GC/MS)
3.2. UV-Visible-Near Infrared Spectroscopy (UV-Vis-NIR)
3.3. Fourier Transform Infrared Spectroscopy (FT-IR)
3.4. Differential Scanning Calorimetry (DSC)
3.5. Thermogravimetric Analysis (TGA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czanderna, A.W.; Pern, F.J. Encapsulation of PV modules using ethylene vinyl acetate copolymer as a pottant: A critical review. Sol. Energy Mater. Solar Cells 1996, 43, 101–181. [Google Scholar] [CrossRef]
- Ndiaye, A.; Charki, A.; Kobi, A.; Kébé, C.M.; Ndiaye, P.A.; Sambou, V. Degradations of silicon photovoltaic modules: A literature review. Sol. Energy 2013, 96, 140–151. [Google Scholar] [CrossRef]
- Kempe, M. Encapsulant Materials for PV Modules. In Photovoltaic Solar Energy: From Fundamentals to Applications; Reinders, A., Verlinden, P., van Sark, W., Freundlich, A., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 478–490. [Google Scholar] [CrossRef]
- Omazic, A.; Oreski, G.; Halwachs, M.; Eder, G.C.; Hirschl, C.; Neumaier, L.; Pinter, G.; Erceg, M. Relation between degradation of polymeric components in crystalline silicon PV module and climatic conditions: A literature review. Sol. Energy Mater. Solar Cells 2019, 192, 123–133. [Google Scholar] [CrossRef]
- Badiee, A.; Ashcroft, I.A.; Wildman, R.D. The thermo-mechanical degradation of ethylene vinyl acetate used as a solar panel adhesive and encapsulant. Int. J. Adhes Adhes 2016, 68, 212–218. [Google Scholar] [CrossRef]
- Pern, F.J. Factors that affect the EVA encapsulant discoloration rate upon accelerated exposure. Sol. Energy Mater. Solar Cells 1996, 587–615. [Google Scholar] [CrossRef]
- Pern, F.J.; Czanderna, A.W. Characterization of ethylene vinyl acetate (EVA) encapsulant: Effects of thermal processing and weathering degradation on its discoloration. Sol. Energy Mater. Solar Cells 1992, 25, 3–23. [Google Scholar] [CrossRef]
- Oreski, G.; Ottersböck, B.; Omazic, A. 6-Degradation Processes and Mechanisms of Encapsulants. In Durability and Reliability of Polymers and Other Materials in Photovoltaic Modules; Yang, H.E., French, R.H., Bruckman, L.S., Eds.; William Andrew Publishing: New York, NY, USA, 2019; pp. 135–152. ISBN 978-0-12-811545-9. [Google Scholar]
- Weber, U.; Eiden, R.; Strubel, C.; Soegding, T.; Heiss, M.; Zachmann, P.; Nattermann, K.; Engelmann, H.; Dethlefsen, A.; Lenck, N. Acetic Acid Production, Migration and Corrosion Effects in Ethylene-Vinyl-Acetate- (EVA-) Based PV Modules. Presented at 27th European Photovoltaic Solar Energy Conference and Exhibition, Frankfurt, Germany, 24–28 September 2012; pp. 2992–2995. [Google Scholar]
- Matsuda, K.; Watanabe, T.; Sakaguchi, K.; Yoshikawa, M.; Doi, T.; Masuda, A. Microscopic Degradation Mechanisms in Silicon Photovoltaic Module under Long-Term Environmental Exposure. Jpn. J. Appl. Phys. 2012, 51, 10NF07. [Google Scholar] [CrossRef]
- Jonai, S.; Hara, K.; Tsutsui, Y.; Nakahama, H.; Masuda, A. Relationship between cross-linking conditions of ethylene vinyl acetate and potential induced degradation for crystalline silicon photovoltaic modules. Jpn. J. Appl. Phys. 2015, 54, 08KG01. [Google Scholar] [CrossRef]
- Peike, C.; Purschke, L.; Weiß, K.-A.; Köhl, M.; Kempe, M. Towards the origin of photochemical EVA discoloration. Presented at 39th Photovoltaic Specialists Conference IEEE, Tampa, FL, USA, 16–21 June 2013. [Google Scholar]
- Chang, J.; Yang, H.; Wang, H.; Cao, D. The investigation of snail trails in photovoltaic modules. Presented at IEEE 42nd Photovoltaic Specialist Conference, New Orleans, LA, USA, 12–19 June 2015. [Google Scholar]
- Jentsch, A.; Eichhorn, K.-J.; Voit, B. Influence of typical stabilizers on the aging behavior of EVA foils for photovoltaic applications during artificial UV-weathering. Polym. Test. 2015, 44, 242–247. [Google Scholar] [CrossRef]
- López-Escalante, M.; Caballero, L.J.; Martín, F.; Gabás, M.; Cuevas, A.; Ramos-Barrado, J. Polyolefin as PID-resistant encapsulant material in 5PV6 modules. Sol. Energy Mater. Sol. Cells 2016, 144, 691–699. [Google Scholar] [CrossRef]
- Adothu, B.; Bhatt, P.; Chattopadhyay, S.; Zele, S.; Oderkerk, J.; Sagar, H.P.; Costa, F.R.; Mallick, S. Newly developed thermoplastic polyolefin encapsulant–A potential candidate for crystalline silicon photovoltaic modules encapsulation. Sol. Energy 2019, 194, 581–588. [Google Scholar] [CrossRef]
- Adothu, B.; Bhatt, P.; Zele, S.; Oderkerk, J.; Costa, F.R.; Mallick, S. Investigation of newly developed thermoplastic polyolefin encapsulant principle properties for the c-Si PV module application. J. Mater. Chem. Phys. 2020, 243, 122660. [Google Scholar] [CrossRef]
- Ottersböck, B.; Oreski, G.; Pinter, G. Comparison of different microclimate effects on the aging behavior of encapsulation materials used in photovoltaic modules. Polym. Degrad. Stab. 2017, 182–191. [Google Scholar] [CrossRef]
- Oreski, G.; Omazic, A.; Eder, G.; Voronko, Y.; Neumaier, L.; Mühleisen, W.; Hirschl, C.; Ujvari, G.; Eber, R.; Edler, M. Properties and degradation behaviour of polyolefin encapsulants for PV modules. Prog. Photovolt. 2020, 28, 1277–1288. [Google Scholar] [CrossRef]
- Salvalaggio, M.; Bagatin, R.; Fornaroli, M.; Fanutti, S.; Palmery, S.; Battistel, E. Multi-component analysis of low-density polyethylene oxidative degradation. Polym. Degrad. Stab. 2006, 91, 2775–2785. [Google Scholar] [CrossRef]
- Yang, R.; Christensen, P.A.; Egerton, T.A.; White, J.R. Degradation products formed during UV exposure of polyethylene–ZnO nano-composites. Polym. Degrad. Stab. 2010, 95, 1533–1541. [Google Scholar] [CrossRef]
- Abdelhafidi, A.; Chabira, S.; Yagoubi, W.; Mistretta, M.; Lamantia, F.; Sebaa, M.; Benchatti, A. Sun radiation and temperature impact at different periods of the year on the photooxidation of polyethylene films. IJHT 2017, 35, 255–261. [Google Scholar] [CrossRef]
- Eder, G.C.; Voronko, Y.; Oreski, G.; Mühleisen, W.; Knausz, M.; Omazic, A.; Rainer, A.; Hirschl, C.; Sonnleitner, H. Error analysis of aged modules with cracked polyamide backsheets. Sol. Energy Mater. Solar Cells 2019, 203, 110194. [Google Scholar] [CrossRef]
- Annigoni, E.; Virtuani, A.; Caccivio, M.; Friesen, G.; Chianese, D.; Ballif, C. 35 years of photovoltaics: Analysis of the TISO-10-kW solar plant, lessons learnt in safety and performance—Part 2. Prog. Photovolt. 2019, 27, 760–778. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Liauw, C.M.; Allen, N.S.; Edge, M.; Fontan, E. Degradation and stabilization of poly(ethylene-stat-vinyl acetate): 1—Spectroscopic and rheological examination of thermal and thermo-oxidative degradation mechanisms. Polym. Degrad. Stab. 2006, 91, 154–164. [Google Scholar] [CrossRef]
- Yagoubi, W.; Abdelhafidi, A.; Sebaa, M.; Chabira, S.F. Identification of carbonyl species of weathered LDPE films by curve fitting and derivative analysis of IR spectra. Polym. Test. 2015, 44, 37–48. [Google Scholar] [CrossRef]
- Ehrenstein, G.W.; Riedel, G.; Trawiel, P. Thermal Analysis of Plastics. Theory and Practice; Carl Hanser Verlag: Munich, Germany, 2004; ISBN 978-1569903629. [Google Scholar]
- Hirschl, C.; Biebl–Rydlo, M.; DeBiasio, M.; Mühleisen, W.; Neumaier, L.; Scherf, W.; Oreski, G.; Eder, G.; Chernev, B.; Schwab, W.; et al. Determining the degree of crosslinking of ethylene vinyl acetate photovoltaic module encapsulants—A comparative study. Sol. Energy Mater. Solar Cells 2013, 116, 203–218. [Google Scholar] [CrossRef] [Green Version]
- Klemchuk, P.; Ezrin, E.; Lavigne, G.; Holley, W.; Galica, J.; Agro, S. Investigation of the degradation and stabilization of EVA-based encapsulant in field-aged solar energy modules. Polym. Degrad. Stab. 1997, 347–365. [Google Scholar] [CrossRef]
- Hintersteiner, I.; Sternbauer, L.; Beissmann, S.; Buchberger, W.W.; Wallner, G.M. Determination of stabilisers in polymeric materials used as encapsulants in photovoltaic modules. Polym. Test. 2014, 33, 172–178. [Google Scholar] [CrossRef]
- Gugumus, F. Possibilities and limits of synergism with light stabilizers in polyolefins 2. UV absorbers in polyolefins. Polym. Degrad. Stab. 2002, 75, 309–320. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Manzanos, M.J.; Goicoechea, E.; Guillén, M.D. 2,6-Di-Tert-Butyl-Hydroxytoluene and Its Metabolites in Foods. Compr. Rev. Food Sci. Food Saf. 2015, 14, 67–80. [Google Scholar] [CrossRef]
- Miller, D.C.; Bokria, J.G.; Burns, D.M.; Fowler, S.; Gu, X.; Hacke, P.L.; Honeker, C.C.; Kempe, M.D.; Köhl, M.; Phillips, N.H.; et al. Degradation in photovoltaic encapsulant transmittance: Results of the first PVQAT TG5 artificial weathering study. Prog. Photovolt. 2019, 27, 391–409. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies. Tables and Charts, 3rd ed.; John Wiley & Sons, Ltd.: Chichester, West Sussex, UK, 2001. [Google Scholar]
- Allen, N.S.; Edge, M.; Rodriguez, M.; Liauw, C.M.; Fontan, E. Aspects of the thermal oxidation, yellowing and stabilisation of ethylene vinyl acetate copolymer. Polym. Degrad. Stab. 2001, 71, 1–14. [Google Scholar] [CrossRef]
- Gulmine, J.V.; Janissek, P.; Heise, H.; Akcelrud, L. Degradation profile of polyethylene after artificial accelerated weathering. Polym. Degrad. Stab. 2003, 79, 385–397. [Google Scholar] [CrossRef]
- Schlothauer, J.C.; Grabmayer, K.; Hintersteiner, I.; Wallner, G.M.; Röder, B. Non-destructive 2D-luminescence detection of EVA in aged PV modules: Correlation to calorimetric properties, additive distribution and a clue to aging parameters. Sol. Energy Mater. Sol. Cells 2017, 159, 307–317. [Google Scholar] [CrossRef]
- Ehrenstein, G.W.; Pongratz, S. Resistance and Stability of Polymers; Hanser Publishers: Munich, Germany, 2013. [Google Scholar]
- Sharma, B.K.; Desai, U.; Singh, A.; Singh, A. Effect of vinyl acetate content on the photovoltaic-encapsulation performance of ethylene vinyl acetate under accelerated ultra-violet aging. J. Appl. Polym. Sci. 2020, 137, 48268. [Google Scholar] [CrossRef]
- Brogly, M.; Nardin, M.; Schultz, J. Effect of Vinylacetate Content on Crystallinity and Second-Order Transitions in Ethylene–Vinylacetate Copolymers. J. Appl. Polym. Sci. 1997, 64, 1903–1912. [Google Scholar] [CrossRef]
- White, J.R. Polymer ageing: Physics, chemistry or engineering? Time to reflect. Comptes Rendus Chim. 2006, 9, 1396–1408. [Google Scholar] [CrossRef]
- Fairbrother, A.; Hsueh, H.-C.; Kim, J.H.; Jacobs, D.; Perry, L.; Goodwin, D.; White, C.; Watson, S.; Sung, L.-P. Temperature and light intensity effects on photodegradation of high-density polyethylene. Polym. Degrad. Stab. 2019, 165, 153–160. [Google Scholar] [CrossRef]
- Fayolle, B.; Colin, X.; Audouin, L.; Verdu, J. Mechanism of degradation induced embrittlement in polyethylene. Polym. Degrad. Stab. 2007, 92, 231–238. [Google Scholar] [CrossRef]
















| Encapsulant | Thickness (µm) | Chemical Crosslinking | Acetic Acid |
|---|---|---|---|
| EVA | 450 | Yes, with peroxides | Yes |
| TPO | 500 | No | No |
| POE | 550 | Yes, with peroxides | No |
| Function | Irradiation (W m−2 nm−1) | Black Panel Temperature (°C) | Time (Hours:Minutes) |
|---|---|---|---|
| UV light | 0.76 | 60 | 8:00 |
| Condensation | n/a | 50 | 4:00 |
| EVA | |||||
| Stabilizer | Unexposed | DH Ageing Time 3300 h | UV Dose 85 kW h m−2 | UV Dose 127 kW h m−2 | UV Dose 200 kW h m−2 |
| Antioxidant butylated hydroxytoluene (BHT) | ✓ | ✓ | ✓ | ✓ | n. d. |
| UV absorber (benzophenone) | ✓ | ✓ | ✓ | ✓ | ✓ |
| UV absorber (benzotriazole) | n. d. | ✓ | n. d. | n. d. | n. d. |
| TPO | |||||
| Stabilizer | Unexposed | DH ageing time 3300 h | UV dose 85 kW h m−2 | UV dose 127 kW h m−2 | UV dose 200 kW h m−2 |
| Antioxidant (Antioxidant 1076) | ✓ | fragment | n. d. | n. d. | n. d. |
| UV absorber (benzotriazole) | n. d. | ✓ | n. d. | n. d. | n. d. |
| POE | |||||
| Stabilizer | Unexposed | DH ageing time 3300 h | UV dose 85 kW h m−2 | UV dose 127 kW h m−2 | UV dose 200 kW h m−2 |
| Antioxidant (BHT) | ✓ | fragment | n. d. | n. d. | n. d. |
| UV absorber (benzotriazole) | n. d. | ✓ | n. d. | n. d. | n. d. |
| Antioxidant (Antioxidant 1076) | n. d. | n. d. | traces | traces | traces |
| Wavenumber [cm−1] | Assignment |
|---|---|
| 2920 | Asymmetric stretching vibration of CH2 |
| 2850 | Symmetric deformation vibration of CH2 |
| 1780 | C=O stretching vibration of γ-lactones |
| 1715/1175 | C=O stretching vibration of ketones |
| 1736 | C=O stretching vibration |
| 1465 | Asymmetric deformation vibration of CH2 |
| 1370 | Symmetric deformation of CH3 |
| 1238 | C-O-C stretching vibration |
| 1020 | C-O-C stretching vibration |
| 960–940 | CH out-of-plane deformation vibration of vinyl ether |
| 910 | CH out-of-plane deformation vibration of vinyl |
| 720 | CH2 skeleton rocking vibration |
| Wavenumber [cm−1] | Assignment |
|---|---|
| 2920 | Asymmetric stretching vibration of CH2 |
| 2850 | Symmetric stretching vibration of CH2 |
| 1800–1680 | C=O stretching vibration |
| 1715/1175 | C=O stretching vibration of ketones |
| 1465 | Asymmetric deformation vibration of CH2 |
| 1370 | Symmetric deformation of CH3 |
| 909 | CH out-of-plane deformation vibration of vinyl |
| 720 (doublet) | CH2 skeleton rocking vibration |
| EVA | POE | TPO | |
|---|---|---|---|
| Chemical crosslinking | Yes | Yes | No |
| Acetic acid | Yes | No | No |
| General DH stability after 3300 h | Very good | Very good, transmittance decreases in UV range | Very good, transmittance decreases in UV range |
| Presence of stabilizers upon UV exposure | Yes | Partial | No |
| Optical properties upon UV exposure | Slight transmittance decrease | Slight transmittance decrease | Not measurable |
| Chemical oxidation upon UV exposure | Initial stage | Initial stage | Severe |
| Crystallinity changes upon UV exposure | Not relevant | Not relevant | Yes |
| Thermal stability upon UV exposure | Decreased | Decreased | Very much decreased |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barretta, C.; Oreski, G.; Feldbacher, S.; Resch-Fauster, K.; Pantani, R. Comparison of Degradation Behavior of Newly Developed Encapsulation Materials for Photovoltaic Applications under Different Artificial Ageing Tests. Polymers 2021, 13, 271. https://doi.org/10.3390/polym13020271
Barretta C, Oreski G, Feldbacher S, Resch-Fauster K, Pantani R. Comparison of Degradation Behavior of Newly Developed Encapsulation Materials for Photovoltaic Applications under Different Artificial Ageing Tests. Polymers. 2021; 13(2):271. https://doi.org/10.3390/polym13020271
Chicago/Turabian StyleBarretta, Chiara, Gernot Oreski, Sonja Feldbacher, Katharina Resch-Fauster, and Roberto Pantani. 2021. "Comparison of Degradation Behavior of Newly Developed Encapsulation Materials for Photovoltaic Applications under Different Artificial Ageing Tests" Polymers 13, no. 2: 271. https://doi.org/10.3390/polym13020271
APA StyleBarretta, C., Oreski, G., Feldbacher, S., Resch-Fauster, K., & Pantani, R. (2021). Comparison of Degradation Behavior of Newly Developed Encapsulation Materials for Photovoltaic Applications under Different Artificial Ageing Tests. Polymers, 13(2), 271. https://doi.org/10.3390/polym13020271