1. Introduction
Foaming in thermoplastic polymers has attracted attention, since it provides the produced materials with a combination of desirable properties like thermal insulation and high specific strength, with low density [
1]. Different foaming approaches like mechanical physical and chemical have been used [
2,
3,
4]. The polymeric foams are also known as porous polymeric material, as they consist of a polymeric matrix, which contains an important number of small pores. Polymer foams can develop a different geometry, such as open cells or closed cells, depending on the raw materials and the method used for their creation. Polymeric foams—because of their advantages—can fit in different fields, such as packaging, thermal insulation systems, sporting equipment and automotive parts [
5,
6]. Polymeric foams find applications also in food packaging, providing lightweight insulating properties to the package [
7]. The most commonly used polymeric foam used for food packaging is expanded polystyrene (EPS). EPS however poses environmental concerns since it is not degradable and is difficult to recycle [
8].
Polyolefins are among the polymers used widely for food packaging applications and are considered as promising polymers for foaming because of their low cost and their recycling ability, as they are used in a variety of industrial applications. Polypropylene (PP) is one of the most popular thermoplastic materials. However, PP due to its linear structure and low melt strength shows difficulties in the foaming processing. For this reason, foaming of polypropylene is very challenging and a combination of physical and chemical foaming agents are required in some cases [
6]. Furthermore, in cases that applications in food packaging are targeted, only foaming agents that are approved for foods can be used (e.g., EC 1935/2004 [
9]). Such materials are inorganic compounds, such as sodium bicarbonate and their combination with organic acids which havedecomposition temperatures that are compatible with the polypropylene processing temperature [
10,
11,
12,
13,
14]. Chemical foaming is an important foaming method and it is important to choose the range of the decomposition temperature of the foaming agent to match the processing temperature of the matrix polymer. The decomposition products of chemical blowing agents, which can be used in different temperature ranges, are various gases such as CO
2 or N
2. For material designed for contact with food, the choice of chemical blowing agents is limited. The most commonly used chemical agents in this case are various food ingredients, like fatty acids, vegetable fats, fatty esters, talc, and sodium bicarbonate. Sodium bicarbonate decomposes at a low temperature (145–150 °C) and carbon dioxide is released [
14], while its combination with the fatty acids and esters having melting points from 50 to 200 °C is expected to influence the gas evolution.
However, introduction of sodium bicarbonate and organic acids in powder form find limitations in injection molding of polypropylene, due to inability to produce the proper master batches since their decomposition process is completed at the PP processing temperature. As an alternative approach coating of PP pellets was attempted using a water-based system using polyethylenoxide (PEO) as the supporting agent. Furthermore, coating of PP pellets was performed using lipid systems.
Another challenge for producing PP foams by injection molding is the type of technology used. Whereas new injection molding technologies have been introduced for the production of polymer foams [
15,
16,
17], the use of standard injection molding technology without any modification can be a challenge for the production of such material [
15]. Making use of modified injection molding machines and chemical blowing agents, which cannot be used to produce material intended for contact with food, it has been possible to produce foams with high void fractions [
17,
18,
19,
20]. Void fractions in this case reach even 60% [
18]. Such blowing agents include azodicarbonamide which has been prohibited for use in foods and in packaging materials intended to come into contact with food in many countries including the EU since 2005 [
21]. Using world-wide authorized food grade chemical blowing agents and modified injection molding methods, it has also been possible to reach significant void fractions for relatively thick parts (36% for material thicknesses of 2.6 mm) [
22]. Nevertheless, to the best of the authors’ knowledge, it is not known whether it would be possible to produce PP foams using typical injection molding machines and food grade ingredients and decrease significantly PP mass or confer thermal insulation properties to the package.
In the present work, polypropylene (PP) was combined with food grade foaming agents like sodium bicarbonate and fatty acids and esters. For that combination, two coating methodologies were followed using either polyethylenoxide (PEO) or fatty esters as the binding factors for the PP pellets coating stabilization. Furthermore, PP compositions with certain amounts of sodium bicarbonate and different fatty acids or esters were used for the production of the foamed PP based products. Initially a laboratory batch injection machine was used and material with selected compositions, presenting a promising behavior, in terms of pore formation, were transferred to industrial injection processing. The obtained articles and products were characterized in respect to the porous formation using microscopic techniques like SEM and CLSM, and composition/thermal characterization using ATR-FTIR and TGA. Finally, their thermal insulation properties were tested and the void fraction created in the polymer matrix was determined.
3. Results
3.1. Coating with Individual Ingredients
Coating of PP pellets with sodium bicarbonate was attempted using different coating methodologies. These methodologies used either PEO or food grade lipids carriers of NaHCO3 to form the coating. For the PEO approach the following coating methodology was used. Polymers in pellet form like polypropylene pellets were combined with aqueous solutions of NaHCO3 and polyethylene oxide (PEO) that was used in order to improve the adhesion of NaHCO3 to the polymer pellets and stabilize the coating. For the improvement of uniformity of the coating on the pellets different molecular weights (MW = 200,000, 300,000, 600,000, 1,000,000 and 4,000,000) of the polyethylene oxide were studied. The best coating was obtained when the water soluble polymer (PEO) had molecular weight MW = 1,000,000. Initial experiments were performed in a rotary evaporator and in second stage a cylindrical vessel equipped with air inlet and outlet was used that was rotating on a mill rotating system at room temperature. Finally, the resulted coated pellets were dried at 40 °C.
For the lipid film approach, the most effective approach for the formation of lipid coating was the direct dispersion of finely ground NaHCO3 in a palm oil/n-heptane mixture. Heptane was subsequently evaporated under vacuum with simultaneous rotational mixing, then lipid partial crystallization took place to temperature decrease from 70 °C to room temperature to form the final coating. The addition of n-heptane aided to decrease the lipid phase viscosity. This was important in order to homogeneously disperse NaHCO3 at temperatures much lower than its decomposition and chiefly to form a more uniform and thin film during partial lipid crystallization along the evaporation-coating process. Of course, n-heptane could be substituted with hexane or another economic organic solvent in an industrial process but n-heptane was chosen herein for the safety of personnel and for a better control of the sample preparation steps due to its relatively low volatility at room temperatures.
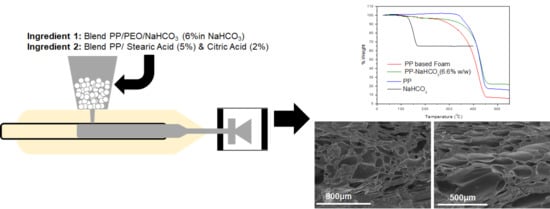
3.2. Combination of Sodium Bicarbonate with Fatty Acids
In order to enhance the gas formation from the sodium bicarbonate decomposition, its combination with fatty acids and other food-grade organic acids was examined. Different additives combinations were tested in various weight ratios. Representative results are shown in
Figure 1 and
Table 2.
SB: NaHCO3 → Na2CO3 + H2O + CO2
CA: C6H8O7 → C6H6O6 + H2O
Mixture SB/CA: C6H8O7 + 3NaHCO3 → C6H8Na3O7 2H2O + 3CO2 + H2O
Additives such as organic acids, are food ingredients and were used for the best foaming of NaHCO
3 (SB). For CO
2 releasing systems the citric acid (CA) is in many cases an endothermic, water soluble acid of choice. The mixture NaHCO
3/citric acid (1/1) has been previously studied [
10]. Decomposition of both compounds and reaction between them is expected to take place in the range of temperatures used during PP processing. Reactions, like the one shown below, lead to carbon dioxide and water vapors generation leading to a 50% weight loss of its original mass after thermal decomposition.
On the other hand, the NaHCO3/stearic acid system decomposed in a narrow temperature range but maintained 80% of its original mass (green line) as opposed to the other two systems that retain approximately 40% of their original mass. Matching of the decomposition characteristic of these systems with the polypropylene process temperature and other process conditions like time and pressure have to be considered for the application to large-scale systems.
3.3. Coating with Combined Ingredients
Based on the above results, systems containing combined ingredients were developed both using PEO and lipids as carriers in different systems. The PEO based approach for the combined coating of PP pellets with an organic acid along with NaHCO3 was used. In this case, citric acid was rejected because it is water-soluble, so it can react with NaHCO3 during the coating process. Thus, stearic acid was chosen, since this remains insoluble in the water and can be incorporated in the coating without reacting with sodium bicarbonate.
The lipid-based approach consisted of replacing palm oil with stearic acid in order to form a stearic acid/NaHCO3 coating. Since an aqueous phase was not used in this approach systems containing NaHCO3 and citric acid or both citric acid and stearic acid were developed using the procedure described above.
3.4. Examination of the Foaming Compositions in Laboratory Scale
Having the above results in mind, the systems NaHCO
3/citric acid, NaHCO
3/stearic acid, NaHCO
3/stearic acid/citric acid coated individually on PP pellets as well as their combination with fatty esters were chosen since their decomposition is completed in temperatures lower that the polypropylene processing temperature. The two selected acids differ on their melting points from 70 °C for stearic to 153 °C for citric acid their 1 to 1 ratio to the sodium bicarbonate gives high degree of gases formation up to 50 wt% as shown in
Table 2. The introduction of fatty esters is expected to influence the PP melt flow and gases evolution and subsequently the pore formation.
Initial experiments, in order to examine the effectiveness of the coatings, were performed in a laboratory injection machine. The different compositions of PP pellets individually or combined coated with the certain amounts of sodium bicarbonate and acids were placed into the heated barrel at a standard temperature of 240 °C. These blends were melted for different time intervals from 1 to 3 min and then injected into a mold under pressure. The different studied systems are gathered in
Table 3. More specifically, the concentration of NaHCO
3 varied from 1.3 to 2%
w/
w, the citric acid concentration from 1 to 2%
w/
w and the stearic acid content from 0.5. to 1%
w/
w. In the case were acid blends were used the total acid concentration varied from 0.5 to 2%
w/
w at a constant citric/stearic acid weight ratio of 2/5. The resulting objects were examined using microscopic analysis (SEM and CLSM) in order to evaluate the porous structure formation. Different retention times were tested in order to find the optimum conditions for the formation of the foamed samples.
Figure 2 presents microscopy results on the porous structure of selected samples with pores ranging from approximately 1 μm to a few hundred micrometers.
The best results were obtained for S1, where both acids were used in combination with NaHCO
3, both at a concentration of 2% (
Figure 2a–c). Reducing the concentration of acids in the blend resulted in an increased time for foaming and the formation of larger pores at lower void fractions without a homogeneous pore structure (S2.
Figure 2d,e). Further reducing the acid concentration resulted in the formation of no voids at all under the same experimental conditions with S1 and S2 (not shown). The effect of the acid type on foam formation can be observed by comparing the results of Sample S4 to S5 (
Figure 2e,f, respectively). The addition of citric acid induced foaming earlier during processing. Furthermore, a more homogeneous pore structure and higher void fractions was obtained by citric acid addition, the latter being in line with the results of TGA (
Figure 1,
Table 2). Furthermore, the overall composition affected the time when the onset of foaming was observed in the samples: samples with a higher concentration in fatty acids and fatty esters (Samples S4, S5, S7) presented lower foam formation times. This was probably due to the effect of fatty esters and fatty acids on the onset of melting and on the melt viscosity which brought NaHCO3 and the acids earlier into contact with one another.
Based on that preliminary examination, compositions that showed foaming in the laboratory batch extruder were prepared in 1–2 kg scale in order to be examined in an industrial scale. The results for these systems are presented in
Section 3.3.
3.5. Examination of the Foaming Compositions in Industrial Scale
3.5.1. Effect of Scale-up and First Screening of Process Variables on Pore Formation
The most successive compositions according to the results of small-scale experiments were examined in large scale. Changing the scale in the experiments induces a significant change in the variables under which the additives decompose and/or react. Namely, the time–temperature profile is significantly different, with process time being approximately ten times lower compared to the small-scale experiments. Furthermore, mixing conditions are different with more intense mixing and high shear stresses in the large-scale experiments. Finally, a very important variable that is expected to affect not only the reactions but also bubble retention and distribution in the low viscosity polymer melt is pressure.
The first consequence of the scale-up (and the consequent change in the process variables) was the significant reduction in the void fraction or the absence of pore formation, even for samples that, in the laboratory scale experiments, showed high void fractions. Therefore, it was considered significant to examine the effect of process variables on the characteristics of the polymer foam. The process variables examined were the injection speed and pressure, the screw rotation speed, the temperature of the mold and oven as well as the residence time and temperature in the mold. These first experiments were performed according to the variables presented in
Table 4.
Figure 3 presents indicative results on the effect of process variables on the microstructure characteristics of the foams. It is observed that increasing the process (oven) temperature from 220 to 240 °C induced an increase in bubble formation (compare
Figure 3a,b) for the same material composition.
Figure 3b–d presents the effect of injection speed on indicative samples. From the samples presented in
Figure 3, as well as from the rest of the samples, it was observed that the injection speed was a significant process variable, which had an effect on the bubble size, void fraction and distribution within the polymer mass, whereas the effect (e.g., increase or decrease in bubble sizes) and the intensity of the effect depended on the rest of variables. It should be noted that, within the range of process variables used, no changes in the microstructure of control PP were observed. As can be observed in
Table 4, both bowls and caps were formed under varying conditions.
Figure 4 presents results on the samples produced using PP coated with NaHCO
3 and organic acids as additives. The void fractions of the bowls and caps were estimated by measuring the weight ratio of the samples vs. a control void fractions for the caps ranged from, 1 to 10% and for the bowls up to 5%. The reasons for the differences between caps and bowls lay not only on the differences in the process variables (rotation speed, pressure etc.) that had to be used in order to form the different parts but probably also on the different thickness of the parts allowing more space for the foam to develop in the thicker caps. Among the process variables that were used for the production of different samples in this set of experiments, the ones that showed a significant effect were the injection speed, the screw rotation speed and the oven and mold temperature.
The resulting samples were tested using Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (ATR-FTIR) and Thermogravimetric analysis (TGA) in order to check if the sodium carbonate had decomposed or not during the production process. The samples were also tested for their mechanical properties by tensile tests and for their morphology was studied by Scanning Electron Microscope (SEM) after fracture in liquid nitrogen.
Results of ATR-FTIR showed no difference between the surface composition of the pure PP packaging and of the one with NaHCO
3 and organic acids for both PEO coated and lipid coated systems (
Figure 5). Due to the fact that the ATR-FTIR analysis focuses on the surface of the sample, the presence of deposited NaHCO
3 on the raw materials is confirmed, as it can be seen but the amount of unreacted NaHCO
3 in the final products cannot be detected through this technique. For that reason, the samples were studied through thermogravimetric analysis (TGA). The results showed that, in several cases, the NaHCO
3 remains unreacted probably due to the very short time that the process takes place (
Figure 6).
3.5.2. Effect of Composition and Fine Tuning of Industrial Scale Process Variables on Pore Formation and Thermal Properties
The experiments and measurements presented in
Section 2.3.1 helped at defining optimum conditions for injection molding in order to form pores within the PP matrix. Given these variables, new experiments were performed where a change in the composition as well as an extensive change in the range of variables (refer to
Table 1) was applied in order to increase the void fraction within the PP matrix. Critical properties like void formation and insulation ability were tested thereafter.
The interest in formation of bubbles and voids in the mass of polymeric packaging material can prove advantageous since bubbles can confer (a) a decrease in the use of polymeric material mass and (b) an increase the insulation properties of packaging materials. To this end, for samples with the best bubble formation according to the microscopy techniques, the void fractions and heat transfer parameters were also determined.
Figure 7 presents SEM and CLSM results as well as the time-temperature profiles obtained at the wall of the cups after filling them with hot water. The compositions and processing conditions used for producing these samples are presented in
Table 5. The temperature differences obtained when hot water was poured in a control package compared to the foamed one for our experiments reached 6.5 °C.
Figure 8 presents the void fractions obtained in the foamed bowls, which were calculated using weight measurements of control samples and foamed ones. Herein, foam formation was represented in terms of void fraction (instead of density or specific gravity) because the new packages included in many cases materials that do not decompose and have significant differences in density. The void fractions obtained for the samples developed in this work reached 14%, which can be considered satisfactory given the type of material, process and package thickness. The above packages presented lower thermal conductivities with respect to the pure PP sample (
Table 6). The observed decrease in thermal conductivity reached 20% for Sample 17b. For the same sample, the thermal conductivity (
Table 6) was close to this of thermal insulation material (<0.1 W/m·K [
23]). Comparing the results of the thermal conductivity with the ones of void fraction, it can be understood that thermal insulation in the packages was not conferred only by the void fraction. Samples S17 and S18, which presented the lowest thermal conductivities, were produced in addition of talc which seems to have affected the thermal conductivity. Regarding void formation, it was observed from the SEM/CLSM images (but also macroscopically) that the bottom of the cup had the larger pores and higher void fractions. A reason for this could be the differences in shear during the filling of the bowls, which can be expected to affect bubble coalescence and their removal out of the package mass. Such a result can also be related to the relatively low temperature differences (temperatures were measured at the walls) between control samples and foamed ones. In addition, void fractions in the caps were up to 200% higher than the bowls, underlining the significance of process conditions. In the developing foams during injection, molding the injection process had a great effect on the number of pores of the final product since a part of the gas was expelled from the PP mass during injection, as shown from the diameter of the extrudate shown in
Figure 9.
In order to reduce the effect of expelling the formed gas from the mold with subsequent reduction of void faction, the piston displacement was limited to a distance so as to allow bubbles within the polymer matrix and produce a fully developed bowl at the same time. The effect of piston displacement can be observed by comparing the void fractions of samples S8 and S9 as well as the ones of S15 and S16 (
Figure 8). The cooling time was another significant variable in foam formation, allowing for foam to develop within the mold. The effect of cooling time on the void fraction can be observed by comparing samples S9 and S10. Furthermore, the increase in oven and mold temperature resulted in an increase in void fractions due to the increase in decomposition/reaction rates (compare samples S14 and S15). This was observed also in preliminary large-scale experiments.
Talc was used in some of the samples in order to observe its potential filler and/or nucleating agent effect. Furthermore, talc increases the viscoelasticity of the polymer melt, thus allowing the formed gas to be better entrapped in the polymer matrix [
24]. In addition, it aids to the mechanical properties of polymer foams [
25]. Among the samples with the highest void fractions were those containing talc. However, similar void fractions were obtained for other reactant compositions without talc. More experiments are required in order to elucidate the effect of talc in our systems.
Apart from the challenges of the process (high pressures, low processing times) and the material (PP has a low melt viscosity), another challenge for increasing the void fraction and the insulating properties of the examined material was the low thickness of the package used for the experiments (0.5, 0.6 mm), which did not allow enough space for bubbles to develop. It was shown previously that the thickness of the material plays a significant role in foam development. Increasing the thickness of the material by 18% resulted in an increase in void fraction of 39% in a core-back injection molding application [
22]. Regarding the material properties, changing the structure of PP can lead to a better development and retention of foam [
15]. Such an approach could improve the present results.
4. Conclusions
This work illustrates the efficient coating of polypropylene pellets with food grade coating agents like sodium bicarbonate and organic acids to create foamed polypropylene articles. Effective coating of PP pellets was achieved through stabilization with PEO or lipids as sodium bicarbonate binders. Different combinations of PP with the foaming agents were tested in a laboratory scale injection molding machine and the most promising compositions were transferred to a large-scale industrial extruder.
Several process variables were found to be critical to the overall success of large-scale extrusion. Specifically, increasing the oven and mold temperature resulted in increased reaction rates and increased foaming. Increasing the cooling time allowed for more time for the foam to develop within the mold. The injection speed and screw rotation speed affected shear and mixing with an interaction between them being observed with respect to their effect on foam/void characteristics.
The displacement of the injection piston (at 100% of the displacement used for pure PP or less) had a significant effect on foam formation, leaving more space for the foam to develop in the mold. Overall, the void fraction achieved for the examined compositions and process reached 14% for the 400 μm specimens of the PP samples containing 4% sodium bicarbonate and 4% citric acid as an example, which was related to the reduction of PP amount used for their production. The new packages presented a decreased thermal conductivity by up to 20%. Finetuning of the process, changing the structure of PP and increasing the thickness of the specimen could result in lower-cost foamed food grade PP articles with improved insulating properties.