High-Density Patterned Array Bonding through Void-Free Divinyl Siloxane Bis-Benzocyclobutene Bonding Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. DVS-BCB Pattern Process on a Silicon Wafer
2.2. DVS-BCB Bonding Process
2.3. DVS-BCB Bonding Characterization
2.4. DVS-BCB Bonding Adhesion Analysis
3. Results and Discussion
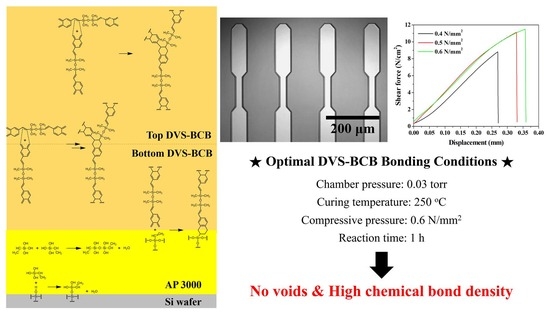
3.1. DVS-BCB Bonding Mechanism
3.2. Characteristics of 3D-Patterned DVS-BCB Bonding Layers
3.3. Adhesive Strength Characteristics of DVS-BCB Bonding
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamineni, V.K.; Singh, P.; Kong, L.; Hudnall, J.; Qureshi, J.; Taylor, C.; Rudack, A.; Arkalgud, S.; Diebold, A.C. Investigation of Optical Properties of Benzocyclobutene Wafer Bonding Layer Used for 3D Interconnects via Infrared Spectroscopic Ellipsometry. Thin Solid Films 2011, 519, 2924–2928. [Google Scholar] [CrossRef]
- Wang, J.; Piskun, I.; Craig, S.L. Mechanochemical Strengthening of a Multi-Mechanophore Benzocyclobutene Polymer. ACS Macro Lett. 2015, 4, 834–837. [Google Scholar] [CrossRef]
- Niklaus, F.; Stemme, G.; Lu, J.-Q.; Gutmann, R.J. Adhesive Wafer Bonding. J. Appl. Phys. 2006, 99, 031101. [Google Scholar] [CrossRef]
- Niklaus, F.; Kumar, R.J.; McMahon, J.J.; Yu, J.; Lu, J.-Q.; Cale, T.S.; Gutmann, R.J. Adhesive Wafer Bonding Using Partially Cured Benzocyclobutene for Three-Dimensional Integration. J. Electrochem. Soc. 2006, 153, G291–G295. [Google Scholar] [CrossRef]
- Zussman, M.P.; Milasincic, C.; Rardin, A.; Kirk, S.; Itabashi, T. Using Permanent and Temporary Polyimide Adhesives in 3D-TSV Processing to Avoid Thin Wafer Handling. Addit. Conf. Device Packag. HiTEC, HiTEN, CICMT 2010, 2010, 859–890. [Google Scholar] [CrossRef]
- Su, G.-D.J.; Toshiyoshi, H.; Wu, M.C. Surface-Micromachined 2-D Optical Scanners with High-Performance Single-Crystalline Silicon Micromirrors. IEEE Photon. Technol. Lett. 2001, 13, 606–608. [Google Scholar] [CrossRef]
- Park, J.; Taweeplengsangsuke, J.; Theis, C.; Osenbach, J. Epoxy Adhesive Used in Optical Fiber/Passive Component: Kinetics, Voids and Reliability. In Proceedings of the 51st Electronic Components and Technology Conference, Orlando, FL, USA, 29 May–1 June 2001; pp. 637–644. [Google Scholar] [CrossRef]
- Kwon, Y.; Jindal, A.; Augur, R.; Seok, J.; Cale, T.S.; Gutmann, R.J.; Lu, J.-Q. Evaluation of BCB Bonded and Thinned Wafer Stacks for Three-Dimensional Integration. J. Electrochem. Soc. 2008, 155, H280–H286. [Google Scholar] [CrossRef]
- Hayes, C.O.; Chen, P.H.; Thedford, R.P.; Ellison, C.J.; Dong, G.; Willson, C.G. Effect of Ring Functionalization on the Reaction Temperature of Benzocyclobutene Thermoset Polymers. Macromolecules. 2016, 49, 3706–3715. [Google Scholar] [CrossRef]
- Wang, S.X.; Zhu, J.; Jia, S.X.; Wu, Y. An Investigation into the High Strength Bonding Technology of Wafer-to-Wafer with Large-Scale Au Line. J. Phys. Conf. Ser. 2019, 1209, 012013. [Google Scholar] [CrossRef] [Green Version]
- Mills, M.E.; Townsend, P.; Castillo, D.; Martin, S.; Achen, A. Benzocyclobutene (DVS-BCB) Polymer as an Interlayer Dielectric (ILD) Material. Microelectron. Eng. 1997, 33, 327–334. [Google Scholar] [CrossRef]
- Iervolino, F.; Suriano, R.; Scolari, M.; Gelmi, I.; Castoldi, L.; Levi, M. Inkjet Printing of a Benzocyclobutene-Based Polymer as a Low-k Material for Electronic Applications. ACS Omega 2021, 6, 15892–15902. [Google Scholar] [CrossRef]
- Quellmalz, A.; Wang, X.; Sawallich, S.; Uzlu, B.; Otto, M.; Wagner, S.; Wang, Z.; Prechtl, M.; Hartwig, O.; Luo, S.; et al. Large-Area Integration of Two-Dimensional Materials and Their Heterostructures by Wafer Bonding. Nat. Commun. 2021, 12, 917. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Z.; Zhu, T.; Li, Z.; Wang, J.; Cheng, Y. Multi-Benzocyclobutene Functionalized Siloxane Monomers Prepared by Piers-Rubinsztajn Reaction for Low-k Materials. Eur. Polym. J. 2020, 126, 109562. [Google Scholar] [CrossRef]
- Lin, S.L.; Huang, W.C.; Ko, C.T.; Chen, K.N. BCB-to-Oxide Bonding Technology for 3D Integration. Microelectron. Reliab. 2012, 52, 352–355. [Google Scholar] [CrossRef]
- Zhang, A.Z.; Wang, Q.; Karlsson, S.; Kjebon, O.; Schatz, R.; Fonjallaz, P.Y.; Almqvist, S.; Chacinski, M.; Thylén, L.; Berggren, J.; et al. Fabrication of an Electro-Absorption Transceiver with a Monolithically Integrated Optical Amplifier for Fiber Transmission of 40–60-GHz Radio Signals. Semicond. Sci. Technol. 2011, 26, 014042. [Google Scholar] [CrossRef]
- Rimböck, J.; Gasiorowski, J.; Pires, M.; Zenger, T.; Burggraf, J.; Dragoi, V. Adhesive Wafer Bonding Using Ultra-Thin Spray-Coated BCB Layers. ECS Trans. 2020, 98, 67–78. [Google Scholar] [CrossRef]
- Bao, S.; Wang, Y.; Lina, K.; Zhang, L.; Wang, B.; Sasangka, W.A.; Lee, K.E.K.; Chua, S.J.; Michel, J.; Fitzgerald, E.; et al. A Review of Silicon-Based Wafer Bonding Processes, an Approach to Realize the Monolithic Integration of Si-CMOS and III-V-On-Si Wafers. J. Semicond. 2021, 42, 023106. [Google Scholar] [CrossRef]
- Lorenz, N.; Smith, M.D.; Hand, D.P. Wafer-Level Packaging of Silicon to Glass with a BCB Intermediate Layer Using Localised Laser Heating. Microelectron. Reliab. 2011, 51, 2257–2262. [Google Scholar] [CrossRef]
- Song, Z.; Tan, Z.; Liu, L.; Wang, Z. Void-Free BCB Adhesive Wafer Bonding with High Alignment Accuracy. Microsyst. Technol. 2015, 21, 1633–1641. [Google Scholar] [CrossRef]
- Kim, H.S.; Najafi, K. Wafer Bonding Using Parylene and Wafer-Level Transfer of Free-Standing Parylene Membranes. In Proceedings of the Therapeutic 12th International Conference on Solid State Sensors Actuators and Microsystems (Transducers 2003), Boston, MA, USA, 8–12 June 2003; Volume 790–793. [Google Scholar] [CrossRef]
- Chen, P.H.; Lin, C.L.; Liu, C.Y. Amorphous Si/Au Wafer Bonding. Appl. Phys. Lett. 2007, 90, 132120. [Google Scholar] [CrossRef]
- Zhang, X.X.; Raskin, J.-P. Low-Temperature Wafer Bonding: A Study of Void Formation and Influence on Bonding Strength. J. Microelectromech. Syst. 2005, 14, 368–382. [Google Scholar] [CrossRef]
- Wang, C.; Higurashi, E.; Suga, T. Void-Free Room-Temperature Silicon Wafer Direct Bonding Using Sequential Plasma Activation. Jpn. J. Appl. Phys. 2008, 47, 2526–2530. [Google Scholar] [CrossRef]
- Tong, Q.-Y.; Kaido, G.; Tong, L.; Reiche, M.; Shi, F.; Steinkirchner, J.; Tan, T.Y.; Gösele, U. A Simple Chemical Treatment for Preventing Thermal Bubbles in Silicon Wafer Bonding. J. Electrochem. Soc. 1995, 142, L201–L203. [Google Scholar] [CrossRef]
- Kim, B.; Cakmak, E.; Matthias, T.; Jang, E.J.; Kim, J.W.; Park, Y.B. Sub-micron Aligned Cu-Cu Direct Bonding for TSV Stacking. In Proceedings of the 2010 IEEE/SEMI Advanced Semiconductor Manufacturing Conference (ASMC), San Francisco, CA, USA, 11–13 July 2010; Volume 2010, pp. 88–91. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Wang, Y.; Chen, D. A Modified Low-Temperature Wafer Bonding Method Using Spot Pressing Bonding Technique and Water Glass Adhesive Layer. Jpn. J. Appl. Phys. 2018, 57. [Google Scholar] [CrossRef] [Green Version]
- Dow, C. Processing Procedures for Cyclotene 4000 Series Photo BCB Resins DS2100 Puddle Develop Process; Dow Chemical Company: Midland, MI, USA, 2009; pp. 1–10. [Google Scholar]
- Kwon, Y.; Seok, J.; Lu, J.-Q.; Cale, T.S.; Gutmann, R.J. Critical Adhesion Energy of Benzocyclobutene-Bonded Wafers. J. Electrochem. Soc. 2006, 153, G347–G352. [Google Scholar] [CrossRef]
- Song, Z.; Wu, D.; Zhu, H.; Liu, L.; Wang, Z. Void-Formation in Uncured and Partially-Cured BCB Bonding Adhesive on Patterned Surfaces. Microelectron. Eng. 2015, 137, 164–168. [Google Scholar] [CrossRef]
- Bu, F.; Ma, Q.; Wang, Z. Delamination of Bonding Interface Between Benzocyclobutene (BCB) and Silicon Dioxide/Silicon Nitride. Microelectron. Reliab. 2016, 65, 225–233. [Google Scholar] [CrossRef]
- Ohba, K. Overview of Photo-Definable Benzocyclobutene Polymer. J. Photopol. Sci. Technol. 2002, 15, 177–182. [Google Scholar] [CrossRef]
- Gies, A.P.; Spencer, L.; Rau, N.J.; Boopalachandran, P.; Rickard, M.A.; Kearns, K.L.; McDougal, N.T. Thermally Induced Cross-Linking and Degradation Reactions of Benzocyclobutene-Based Polymers. Macromolecules 2017, 50, 2304–2319. [Google Scholar] [CrossRef]
- Hallani, R.K.; Moser, M.; Bristow, H.; Jenart, M.V.C.; Faber, H.; Neophytou, M.; Yarali, E.; Paterson, A.F.; Anthopoulos, T.D.; McCulloch, I. Low-Temperature Cross-Linking Benzocyclobutene Based Polymer Dielectric for Organic Thin Film Transistors on Plastic Substrates. J. Org. Chem. 2020, 85, 277–283. [Google Scholar] [CrossRef]
- Yeo, B.S.; Schmid, T.; Zhang, W.; Zenobi, R. A Strategy to Prevent Signal Losses, Analyte Decomposition, and Fluctuating Carbon Contamination Bands in Surface-Enhanced Raman Spectroscopy. Appl. Spectrosc. 2008, 62, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Sugitani, S.; Matsuzaki, H.; Enoki, T. Characteristics of Organic Film Deposited by Plasma-Enhanced Chemical-Vapor Deposition Using a Benzocyclobutene Resin. J. Vac. Sci. Technol. A 2004, 22, 2373–2378. [Google Scholar] [CrossRef]
- Herth, E.; Zeggari, R.; Rauch, J.Y.; Remy-Martin, F.; Boireau, W. Investigation of Amorphous SiOx Layer on Gold Surface for Surface Plasmon Resonance Measurements. Microelectron. Eng. 2016, 163, 43–48. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.W.; Choe, H.; Shah, M.A.; Lee, D.-G.; Hur, S. High-Density Patterned Array Bonding through Void-Free Divinyl Siloxane Bis-Benzocyclobutene Bonding Process. Polymers 2021, 13, 3633. https://doi.org/10.3390/polym13213633
Kim NW, Choe H, Shah MA, Lee D-G, Hur S. High-Density Patterned Array Bonding through Void-Free Divinyl Siloxane Bis-Benzocyclobutene Bonding Process. Polymers. 2021; 13(21):3633. https://doi.org/10.3390/polym13213633
Chicago/Turabian StyleKim, Nam Woon, Hyeonjeong Choe, Muhammad Ali Shah, Duck-Gyu Lee, and Shin Hur. 2021. "High-Density Patterned Array Bonding through Void-Free Divinyl Siloxane Bis-Benzocyclobutene Bonding Process" Polymers 13, no. 21: 3633. https://doi.org/10.3390/polym13213633
APA StyleKim, N. W., Choe, H., Shah, M. A., Lee, D. -G., & Hur, S. (2021). High-Density Patterned Array Bonding through Void-Free Divinyl Siloxane Bis-Benzocyclobutene Bonding Process. Polymers, 13(21), 3633. https://doi.org/10.3390/polym13213633