Fabrication of 3D Printed Poly(lactic acid)/Polycaprolactone Scaffolds Using TGF-β1 for Promoting Bone Regeneration
Abstract
:1. Introduction
2. Material and Methods
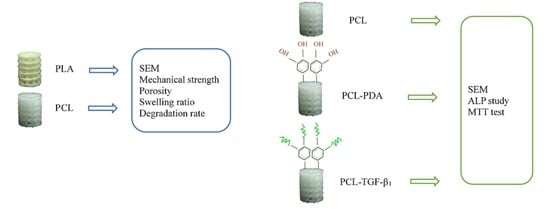
2.1. Creation of 3D Printed PLA and PCL Scaffolds
2.2. Analysis of Physical Property
2.2.1. Mechanical Testing
2.2.2. Measurement of Porosity
2.2.3. Measurement of Swelling Ratio
2.2.4. Degradation Rate Measurements
2.3. Analysis of Biocompatibility In Vitro
Stem Cells Culture by Scanning Electron Microscopy (SEM) Observation
2.4. Coating Poly(dopamine) and Immobilized TGF-β1
2.4.1. Quantification of Immobilized TGF-β1
2.4.2. TGF-β1 Release Test
2.4.3. ALP Assay
2.4.4. Cytotoxicity Testing
3. Results
3.1. Demonstration of PLA and PCL Scaffolds
3.2. Biocompatibility
3.3. Coating PDA and Immobilized TGF-β1
3.3.1. Quantification of Immobilized TGF-β1
3.3.2. TGF-β1 Releasing Test
3.3.3. MG-63 Culture by SEM Observation
3.3.4. MTT Assay with MG-63 Culture
3.3.5. ALP Assay with MG-63 Culture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [Green Version]
- McAllister, B.S.; Haghighat, K. Bone Augmentation Techniques. J. Periodontol. 2007, 78, 377–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Browaeys, H.; Bouvry, P.; De Bruyn, H. A Literature Review on Biomaterials in Sinus Augmentation Procedures. Clin. Implant Dent. Relat. Res. 2007, 9, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Aghaloo, T.L.; Moy, P.K. Which Hard Tissue Augmentation Techniques Are the Most Successful in Furnishing Bony Support for Implant Placement? Int. J. Oral Maxillofac. Implants 2007, 22, 49–70. [Google Scholar]
- Moore, W.R.; Graves, S.E.; Bain, G.I. Synthetic Bone Graft Substitutes. ANZ J. Surg. 2001, 71, 354–361. [Google Scholar] [CrossRef]
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in Orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and Bioactive Porous Polymer/Inorganic Composite Scaffolds for Bone Tissue Engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, M.; Ghasemi, A.; Rolfe, B.; Gibson, I. Additive manufacturing a powerful tool for the aerospace industry. Rapid Prototyp. J. 2021. [Google Scholar] [CrossRef]
- Travieso-Rodriguez, J.A.; Jerez-Mesa, R.; Llumà, J.; Gomez-Gras, G.; Casadesus, O. Comparative study of the flexural properties of ABS, PLA and a PLA–wood composite manufactured through fused filament fabrication. Rapid Prototyp. J. 2021, 27, 81–92. [Google Scholar] [CrossRef]
- Habibovic, P.; Gbureck, U.; Doillon, C.J.; Bassett, D.C.; van Blitterswijk, C.A.; Barralet, J.E. Osteoconduction and Osteoinduction of Low-Temperature 3D Printed Bioceramic Implants. Biomaterials 2008, 29, 944–953. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, C.; Lode, A.; Gelinsky, M. Hierarchical Mesoporous Bioactive Glass/Alginate Composite Scaffolds Fabricated by Three-Dimensional Plotting for Bone Tissue Engineering. Biofabrication 2013, 5, 015005. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Ma, B.; Zhu, H.; Huan, Z.; Ma, N.; Wu, C.; Chang, J. 3D-Printed Bioactive Ca3SiO5 Bone Cement Scaffolds with Nano Surface Structure for Bone Regeneration. ACS Appl. Mater. Interfaces 2017, 9, 5757–5767. [Google Scholar] [CrossRef] [PubMed]
- Montjovent, M.O.; Mathieu, L.; Schmoekel, H.; Mark, S.; Bourban, P.E.; Zambelli, P.Y.; Laurent-Applegate, L.A.; Pioletti, D.P. Repair of Critical Size Defects in the Rat Cranium Using Ceramic-Reinforced PLA Scaffolds Obtained by Supercritical Gas Foaming. J. Biomed. Mater. Res. A 2007, 83, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdal-Hay, A.; Sheikh, F.A.; Lim, J.K. Air Jet Spinning of Hydroxyapatite/Poly(Lactic Acid) Hybrid Nanocomposite Membrane Mats for Bone Tissue Engineering. Colloids Surf. B Biointerfaces 2013, 102, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, C.R.; Shaw, M.T.; Wei, M. Biodegradable HA-PLA 3-D Porous Scaffolds: Effect of Nano-Sized Filler Content on Scaffold Properties. Acta Biomater. 2005, 1, 653–662. [Google Scholar] [CrossRef]
- Jeong, S.I.; Ko, E.K.; Yum, J.; Jung, C.H.; Lee, Y.M.; Shin, H. Nanofibrous Poly(Lactic Acid)/Hydroxyapatite Composite Scaffolds for Guided Tissue Regeneration. Macromol. Biosci. 2008, 8, 328–338. [Google Scholar] [CrossRef]
- Guvendiren, M.; Molde, J.; Soares, R.M.; Kohn, J. Designing Biomaterials for 3D Printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Vidakis, N.; Petousis, M.; Savvakis, K.; Maniadi, A.; Koudoumas, E. A comprehensive investigation of the mechanical behavior and the dielectrics of pure polylactic acid (PLA) and PLA with graphene (GnP) in fused deposition modeling (FDM). Int. J. Plast. Technol. 2019, 23, 195–206. [Google Scholar] [CrossRef]
- Kumar, M.P.; Ponnusamy, S.; Reddy Nallamilli, M.S. The influence of process parameters on the impact resistance of 3D printed PLA specimens under water-absorption and heat-treated conditions. Rapid Prototyp. J. 2021, 27, 1108–1123. [Google Scholar] [CrossRef]
- Afonso, J.A.; Alves, J.L.; Caldas, G.; Gouveia, B.P.; Santana, L.; Belinha, J. Influence of 3D printing process parameters on the mechanical properties and mass of PLA parts and predictive models. Rapid Prototyp. J. 2021, 27, 487–495. [Google Scholar] [CrossRef]
- Von Windheim, N.; Collinson, D.W.; Lau, T.; Brinson, L.C.; Gall, K. The influence of porosity, crystallinity and interlayer adhesion on the tensile strength of 3D printed polylactic acid (PLA). Rapid Prototyp. J. 2021, 27, 1327–1336. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M. Sterilization, Toxicity, Biocompatibility and Clinical Applications of Polylactic Acid/Polyglycolic Acid Copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Mikos, A.G.; Sarakinos, G.; Leite, S.M.; Vacanti, J.P.; Langer, R. Laminated Three-Dimensional Biodegradable Foams for Use in Tissue Engineering. Biomaterials 1993, 14, 323–330. [Google Scholar] [CrossRef]
- Brekke, J.H.; Olson, R.A.; Scully, J.R.; Osbon, D.B. Influence of Polylactic Acid Mesh on the Incidence of Localized Osteitis. Oral Surg. Oral Med. Oral Pathol. 1983, 56, 240–245. [Google Scholar] [CrossRef]
- Levy, F.E.; Hollinger, J.O.; Szachowicz, E.H. Effect of a Bioresorbable Film on Regeneration of Cranial Bone. Plast. Reconstr. Surg. 1994, 93, 307–311. [Google Scholar] [CrossRef]
- Hollinger, J.O. Preliminary Report on the Osteogenic Potential of a Biodegradable Copolymer of Polyactide (PLA) and Polyglycolide (PGA). J. Biomed. Mater. Res. 1983, 17, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Mandakhbayar, N.; El-Fiqi, A.; Lee, J.H.; Kim, H.W. Evaluation of Strontium-Doped Nanobioactive Glass Cement for Dentin-Pulp Complex Regeneration Therapy. ACS Biomater. Sci. Eng. 2019, 5, 6117–6126. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Kishori, B.; Rao, S.; Anjum, M.; Hemanth, V.; Das, S.; Jabbari, E. Electropsun Polycaprolactone Fibres in Bone Tissue Engineering: A Review. Mol. Biotechnol. 2021, 63, 363–388. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable Polymeric Nanoparticles Based Drug Delivery Systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Clover, J.; Gowen, M. Are MG-63 and HOS TE85 human osteosarcoma cell lines representative models of the osteoblastic phenotype? Bone 1994, 15, 585–591. [Google Scholar] [CrossRef]
- Christoph, P.; Matthias, S.; Thomas, T.; Andreas, K.; Peter, N.; Wolf, M.; Stefan, M. Characterization of Osteosarcoma Cell Lines MG-63, Saos-2 and U-2 OS in Comparison to Human Osteoblasts. Anticancer Res. 2004, 24, 3743–3748. [Google Scholar]
- Chia, H.N.; Wu, B.M. Recent Advances in 3D Printing of Biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Asti, A.; Gioglio, L. Natural and Synthetic Biodegradable Polymers: Different Scaffolds for Cell Expansion and Tissue Formation. Int. J. Artif. Organs 2014, 37, 187–205. [Google Scholar] [CrossRef]
- Nijhuis, A.W.; van den Beucken, J.J.; Boerman, O.C.; Jansen, J.A.; Leeuwenburgh, S.C. 1-Step Versus 2-Step Immobilization of Alkaline Phosphatase and Bone Morphogenetic protein-2 onto Implant Surfaces Using Polydopamine. Tissue Eng. Part C Methods 2013, 19, 610–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, H.J.; Perikamana, S.K.; Lee, J.H.; Lee, J.; Lee, K.M.; Shin, C.S.; Shin, H. Effective Immobilization of BMP-2 Mediated by Polydopamine Coating on Biodegradable Nanofibers for Enhanced In Vivo Bone Formation. ACS Appl. Mater. Interfaces 2014, 6, 11225–11235. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Tada, S.; Kitajima, T.; Son, T.I.; Aigaki, T.; Ito, Y. Immobilization of Bone Morphogenetic Protein on DOPA- or Dopamine-Treated Titanium Surfaces to Enhance Osseointegration. BioMed Res. Int. 2013, 2013, 265980. [Google Scholar] [CrossRef]
- Tsai, W.B.; Chen, W.T.; Chien, H.W.; Kuo, W.H.; Wang, M.J. Poly(Dopamine) Coating to Biodegradable Polymers for Bone Tissue Engineering. J. Biomater. Appl. 2014, 28, 837–848. [Google Scholar] [CrossRef]
- Ko, E.; Yang, K.; Shin, J.; Cho, S.W. Polydopamine-Assisted Osteoinductive Peptide Immobilization of Polymer Scaffolds for Enhanced Bone Regeneration by Human Adipose-Derived Stem Cells. Biomacromolecules 2013, 14, 3202–3213. [Google Scholar] [CrossRef]
- Hiyama, A.; Gogate, S.S.; Gajghate, S.; Mochida, J.; Shapiro, I.M.; Risbud, M.V. BMP-2 and TGF-Beta Stimulate Expression of beta1,3-glucuronosyl Transferase 1 (GlcAT-1) in Nucleus Pulposus Cells Through AP1, TonEBP, and Sp1: Role of MAPKs. J. Bone Miner. Res. 2010, 25, 1179–1190. [Google Scholar] [PubMed] [Green Version]
- Kawaguchi, H.; Oka, H.; Jingushi, S.; Izumi, T.; Fukunaga, M.; Sato, K.; Matsushita, T.; Nakamura, K. A Local Application of Recombinant Human Fibroblast Growth Factor 2 for Tibial Shaft Fractures: A Randomized, Placebo-Controlled Trial. J. Bone Miner. Res. 2010, 25, 2735–2743. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Li, Y.; Chen, C.; Xu, Y.; Zang, X.; Li, L.; Meng, K. Experimental Study of the Synergistic Effect and Network Regulation Mechanisms of an Applied Combination of BMP-2, VEGF, and TGF-beta1 on Osteogenic Differentiation. J. Cell. Biochem. 2020, 121, 2394–2405. [Google Scholar] [CrossRef]
- Li, Z.; Leung, M.; Hopper, R.; Ellenbogen, R.; Zhang, M. Feeder-Free Self-Renewal of Human Embryonic Stem Cells in 3D Porous Natural Polymer Scaffolds. Biomaterials 2010, 31, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Yang, K.C.; Lin, K.H.; Liu, H.C.; Lin, F.H. A Highly Organized Three-Dimensional Alginate Scaffold for Cartilage Tissue Engineering Prepared by Microfluidic Technology. Biomaterials 2011, 32, 7118–7126. [Google Scholar] [CrossRef]
- Cheng, C.H.; Chen, Y.W.; Kai-Xing Lee, A.; Yao, C.H.; Shie, M.Y. Development of Mussel-Inspired 3D-Printed Poly (Lactic Acid) Scaffold Grafted with Bone Morphogenetic protein-2 for Stimulating Osteogenesis. J. Mater. Sci. Mater. Med. 2019, 30, 78. [Google Scholar] [CrossRef]
- Mata, A.; Kim, E.J.; Boehm, C.A.; Fleischman, A.J.; Muschler, G.F.; Roy, S. A Three-Dimensional Scaffold with Precise Micro-Architecture and Surface Micro-Textures. Biomaterials 2009, 30, 4610–4617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borden, M.; Attawia, M.; Khan, Y.; Laurencin, C.T. Tissue Engineered Microsphere-Based Matrices for Bone Repair: Design and Evaluation. Biomaterials 2002, 23, 551–559. [Google Scholar] [CrossRef]
- Carter, D.R.; Spengler, D.M. Mechanical Properties and Composition of Cortical Bone. Clin. Orthop. Relat. Res. 1978, 135, 192–217. [Google Scholar] [CrossRef]
- Gibson, L.J. The Mechanical Behaviour of Cancellous Bone. J. Biomech. 1985, 18, 317–328. [Google Scholar] [CrossRef]
- Havaldar, R.; Pilli, S.C.; Putti, B.B. Insights into the Effects of Tensile and Compressive Loadings on Human Femur Bone. Adv. Biomed. Res. 2014, 3, 101. [Google Scholar] [CrossRef] [PubMed]
- Mohebbi-Kalhori, D.; Behzadmehr, A.; Doillon, C.J.; Hadjizadeh, A. Computational Modeling of Adherent Cell Growth in a Hollow-Fiber Membrane Bioreactor for Large-Scale 3-D Bone Tissue Engineering. J. Artif. Organs 2012, 15, 250–265. [Google Scholar] [CrossRef]
- Li, F.; Wang, S.; Liu, W.; Chen, G. [Progress on Biodegradation of Polylactic Acid—A Review]. Wei Sheng Wu Xue Bao 2008, 48, 262–268. [Google Scholar]
- Mackenzie, C.G.; Mackenzie, J.B.; Beck, P. The Effect of pH on Growth, Protein Synthesis, and Lipid-Rich Particles of Cultured Mammalian Cells. J. Biophys. Biochem. Cytol. 1961, 9, 141–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hand, S.; Wang, B.; Chu, K.H. Biodegradation of 1,4-Dioxane: Effects of Enzyme Inducers and Trichloroethylene. Sci. Total Environ. 2015, 520, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Malysheva, K.V.; Spasyuk, I.M.; Pavlenko, O.K.; Stoika, R.S.; Gorchynskyi, O.G. Generation of Optimized Preparations of Bone Morphogenetic Proteins for Bone Regeneration. Ukr. Biochem. J. 2016, 88, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Han, Y.; Li, J.; Cai, B.; Gao, H.; Feng, W.; Li, S.; Liu, J.; Li, D. BMP-2 Immobilized PLGA/Hydroxyapatite Fibrous Scaffold via Polydopamine Stimulates Osteoblast Growth. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.Y.; Tsai, W.B. Poly(Dopamine)-Assisted Immobilization of Arg–Gly–Asp Peptides, Hydroxyapatite, and Bone Morphogenic protein-2 on Titanium to Improve the Osteogenesis of Bone Marrow Stem Cells. ACS Appl. Mater. Interfaces 2013, 5, 6975–6983. [Google Scholar] [CrossRef] [PubMed]
- Ritz, U.; Gerke, R.; Götz, H.; Stein, S.; Rommens, P.M. A New Bone Substitute Developed from 3D-Prints of Polylactide (PLA) Loaded with Collagen I: An In Vitro Study. Int. J. Mol. Sci. 2017, 18, 2569. [Google Scholar] [CrossRef] [Green Version]
- Bittner, S.M.; Guo, J.L.; Mikos, A.G. Spatiotemporal Control of Growth Factors in Three-Dimensional Printed Scaffolds. Bioprinting 2018, 12, e00032. [Google Scholar] [CrossRef]
- Bittner, S.M.; Guo, J.L.; Melchiorri, A.; Mikos, A.G. Three-Dimensional Printing of Multilayered Tissue Engineering Scaffolds. Mater. Today 2018, 21, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Diaz-Gomez, L.; Xie, V.Y.; Bittner, S.M.; Jiang, E.Y.; Wang, B.; Mikos, A.G. Three-Dimensional Printing of Click Functionalized, Peptide Patterned Scaffolds for Osteochondral Tissue Engineering. Bioprinting 2021, 22, e00136. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Melchels, F.P.; Jeon, J.E.; van Bussel, E.M.; Kimpton, L.S.; Byrne, H.M.; Dhert, W.J.; Dalton, P.D.; Hutmacher, D.W.; Malda, J. Reinforcement of Hydrogels Using Three-Dimensionally Printed Microfibres. Nat. Commun. 2015, 6, 6933. [Google Scholar] [CrossRef] [PubMed]
- Jakus, A.E.; Rutz, A.L.; Jordan, S.W.; Kannan, A.; Mitchell, S.M.; Yun, C.; Koube, K.D.; Yoo, S.C.; Whiteley, H.E.; Richter, C.P.; et al. Hyperelastic. ‘Bone’: A Highly Versatile, Growth Factor-Free, Osteoregenerative, Scalable, and Surgically Friendly Biomaterial. Sci. Transl. Med. 2016, 8, 358ra127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.; Chen, M.; Wang, Z.; Tian, Y.; Zheng, J.; Gao, S.; Li, Y.; Zheng, Y.; Li, X.; Huang, J.; et al. 3D-Printed Cell-Free PCL-MECM Scaffold with Biomimetic Micro-Structure and Micro-Environment to Enhance In Situ Meniscus Regeneration. Bioact. Mater. 2021, 6, 3620–3633. [Google Scholar] [CrossRef] [PubMed]
- Sarker, B.; Li, W.; Zheng, K.; Detsch, R.; Boccaccini, A.R. Designing Porous Bone Tissue Engineering Scaffolds with Enhanced Mechanical Properties from Composite Hydrogels Composed of Modified Alginate, Gelatin, and Bioactive Glass. ACS Biomater. Sci. Eng. 2016, 2, 2240–2254. [Google Scholar] [CrossRef]
- Rustom, L.E.; Boudou, T.; Nemke, B.W.; Lu, Y.; Hoelzle, D.J.; Markel, M.D.; Picart, C.; Wagoner Johnson, A.J. Multiscale Porosity Directs Bone Regeneration in Biphasic Calcium Phosphate Scaffolds. ACS Biomater. Sci. Eng. 2017, 3, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Link, D.P.; van den Dolder, J.; van den Beucken, J.J.; Wolke, J.G.; Mikos, A.G.; Jansen, J.A. Bone Response and Mechanical Strength of Rabbit Femoral Defects Filled with Injectable CaP Cements Containing TGF-Beta 1 Loaded Gelatin Microparticles. Biomaterials 2008, 29, 675–682. [Google Scholar] [CrossRef] [Green Version]
- Lamparelli, E.P.; Lovecchio, J.; Ciardulli, M.C.; Giudice, V.; Dale, T.P.; Selleri, C.; Forsyth, N.; Giordano, E.; Maffulli, N.; Della Porta, G. Chondrogenic Commitment of Human Bone Marrow Mesenchymal Stem Cells in a Perfused Collagen Hydrogel Functionalized with hTGF-beta1-Releasing PLGA Microcarrier. Pharmaceutics 2021, 13, 399. [Google Scholar] [CrossRef]
- Yoo, J.U.; Barthel, T.S.; Nishimura, K.; Solchaga, L.; Caplan, A.I.; Goldberg, V.M.; Johnstone, B. The Chondrogenic Potential of Human Bone-Marrow-Derived Mesenchymal Progenitor Cells. J. Bone Jt. Surg. Am. 1998, 80, 1745–1757. [Google Scholar] [CrossRef]
- Huang, J.I.; Zuk, P.A.; Jones, N.F.; Zhu, M.; Lorenz, H.P.; Hedrick, M.H.; Benhaim, P. Chondrogenic Potential of Multipotential Cells from Human Adipose Tissue. Plast. Reconstr. Surg. 2004, 113, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Moyers-Montoya, E.D.; Escobedo-González, R.G.; Vargas-Requena, C.L.; Garcia-Casillas, P.E.; Martínez-Pérez, C.A. Epithelial Growth Factor-Anchored on Polycaprolactone/6-Deoxy-6-Amino-Beta-Cyclodextrin Nanofibers: In Vitro and In Vivo Evaluation. Polymers 2021, 13, 1303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, M.; Yu, B.; Cao, L.; Yang, Q.; Su, J. Nanocalcium-Deficient Hydroxyapatite-Poly (e-Caprolactone)-Polyethylene Glycol-Poly (e-Caprolactone) Composite Scaffolds. Int. J. Nanomed. 2012, 7, 3123–3131. [Google Scholar]
- Mostafavi, A.; Abudula, T.; Russell, C.S.; Mostafavi, E.; Williams, T.J.; Salah, N.; Alshahrie, A.; Harris, S.; Basri, S.M.M.; Mishra, Y.K.; et al. In Situ Printing of Scaffolds for Reconstruction of Bone Defects. Acta Biomater. 2021, 127, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.; Pedram, A.; Sha’ban, M.; Wan, K.Z.W.S.; Belinda, P.; Kien, H.C. Polycaprolactone-Based Scaffolds Facilitates Osteogenic Differentiation of Human Adipose-Derived Stem Cells in a Co-Culture System. Polymers 2021, 13, 597. [Google Scholar]










| Pore Size (μm) | Line Width (μm) | |
|---|---|---|
| 3D printed PLA | 684 ± 66 | 246 ± 34 |
| 3D printed PCL | 682 ± 50 | 277 ± 36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.-H.; Shie, M.-Y.; Lai, Y.-H.; Foo, N.-P.; Lee, M.-J.; Yao, C.-H. Fabrication of 3D Printed Poly(lactic acid)/Polycaprolactone Scaffolds Using TGF-β1 for Promoting Bone Regeneration. Polymers 2021, 13, 3731. https://doi.org/10.3390/polym13213731
Cheng C-H, Shie M-Y, Lai Y-H, Foo N-P, Lee M-J, Yao C-H. Fabrication of 3D Printed Poly(lactic acid)/Polycaprolactone Scaffolds Using TGF-β1 for Promoting Bone Regeneration. Polymers. 2021; 13(21):3731. https://doi.org/10.3390/polym13213731
Chicago/Turabian StyleCheng, Cheng-Hsin, Ming-You Shie, Yi-Hui Lai, Ning-Ping Foo, Mon-Juan Lee, and Chun-Hsu Yao. 2021. "Fabrication of 3D Printed Poly(lactic acid)/Polycaprolactone Scaffolds Using TGF-β1 for Promoting Bone Regeneration" Polymers 13, no. 21: 3731. https://doi.org/10.3390/polym13213731
APA StyleCheng, C.-H., Shie, M.-Y., Lai, Y.-H., Foo, N.-P., Lee, M.-J., & Yao, C.-H. (2021). Fabrication of 3D Printed Poly(lactic acid)/Polycaprolactone Scaffolds Using TGF-β1 for Promoting Bone Regeneration. Polymers, 13(21), 3731. https://doi.org/10.3390/polym13213731