Cellulose Acetate Nanofibers: Incorporating Hydroxyapatite (HA), HA/Berberine or HA/Moghat Composites, as Scaffolds to Enhance In Vitro Osteoporotic Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Material
2.3. Animal and Ethical Approval
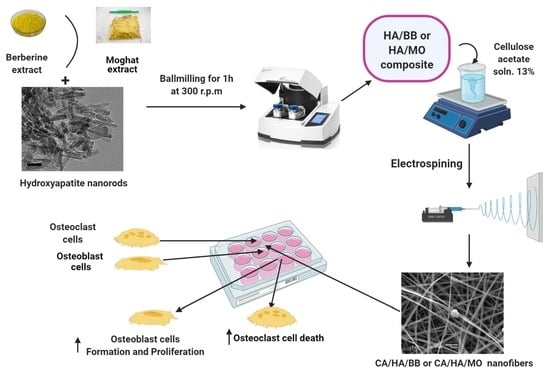
2.4. Preparation of HA/BER and HA/ME Composites Loaded CA Fiber
2.4.1. Preparation of Moghat Extract
2.4.2. Preparation of HA/BER and HA/ME Composites
2.4.3. Preparation of HA, HA/BER, and HA/ME Composites-Loaded Cellulose Acetate Fiber
2.5. Physical Characterization of Scaffolds Samples
2.6. The Mechanical Testing of the Scaffolds
2.7. Water Uptake, Porosity and Contact Angles of the Scaffolds
2.8. Release of Drugs (BER and ME) from HA Composites-Loaded CA13 Fibers
2.9. Isolation of Osteoblast Cells and Induction of Osteoclast Cells Formation
2.10. Cell Viability and Proliferation Test
2.11. Culture of Osteoblast (OB) and Osteoclast (OC) on the Selected Fibers Samples
2.12. Bone Remodelling Biomarkers
2.13. Molecular Investigations
2.14. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, F.-M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [Green Version]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17 (Suppl. 4), 467–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousa, H.M.; Hussein, K.H.; Sayed, M.M.; Abd El-Rahman, M.K.; Woo, H.M. Development and characterization of cellulose/iron acetate nanofibers for bone tissue engineering applications. Polymers 2021, 13, 1339. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Bryant, S.J.; Ahn, J.; Hankenson, K.D. Chapter 24—Bone Regeneration; Atala, A., Allickson, J.G.B.T.-T.R.M., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 313–333. ISBN 978-0-12-410396-2. [Google Scholar]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P. V Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Aoki, K.; Saito, N. Biodegradable Polymers as Drug Delivery Systems for Bone Regeneration. Pharmaceutics 2020, 12, 95. [Google Scholar] [CrossRef] [Green Version]
- Lei, B.; Guo, B.; Rambhia, K.J.; Ma, P.X. Hybrid polymer biomaterials for bone tissue regeneration. Front. Med. 2019, 13, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Bacakova, L.; Novotna, K.; Sopuch, T.; Havelka, P. CELL I NTERACTION WITH C ELLULOSE -B ASED S CAFFOLDS FOR T ISSUE E NGINEERING: A REVIEW. In Cellulose and Cellulose Composites; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2015; ISBN 9781634835534. [Google Scholar]
- Campuzano, S.; Pelling, A.E. Scaffolds for 3D Cell Culture and Cellular Agriculture Applications Derived From Non-animal Sources. Front. Sustain. Food Syst. 2019, 3, 38. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.F.S.; Almeida, F.C.G.; Vinhas, G.M.; Sarubbo, L.A. Production of Bacterial Cellulose by Gluconacetobacter hansenii Using Corn Steep Liquor As Nutrient Sources. Front. Microbiol. 2017, 8, 2027. [Google Scholar] [CrossRef]
- Gupta, P.K. An Update on Overview of Cellulose, Its Structure and Applications; Raghunath, S.S., Ed.; IntechOpen: Rijeka, Croatia, 2019; Chapter 4; ISBN 978-1-83968-057-1. [Google Scholar]
- Hickey, R.J.; Pelling, A.E. Cellulose Biomaterials for Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Tomić, S.; Kokol, V.; Mihajlović, D.; Mirčić, A.; Čolić, M. Native cellulose nanofibrills induce immune tolerance in vitro by acting on dendritic cells. Sci. Rep. 2016, 6, 31618. [Google Scholar] [CrossRef]
- Alfassi, G.; Rein, D.M.; Shpigelman, A.; Cohen, Y. Partially Acetylated Cellulose Dissolved in Aqueous Solution: Physical Properties and Enzymatic Hydrolysis. Polymers 2019, 11, 1734. [Google Scholar] [CrossRef] [Green Version]
- Ohkawa, K. Nanofibers of cellulose and its derivatives fabricated using direct electrospinning. Molecules 2015, 20, 9139–9154. [Google Scholar] [CrossRef] [Green Version]
- Rezk, A.I.; Mousa, H.M.; Lee, J.; Park, C.H.; Kim, C.S. Composite PCL/HA/simvastatin electrospun nanofiber coating on biodegradable Mg alloy for orthopedic implant application. J. Coat. Technol. Res. 2019, 16, 477–489. [Google Scholar] [CrossRef]
- Mousa, H.M.; Tiwari, A.P.; Kim, J.; Adhikari, S.P.; Park, C.H.; Kim, C.S. A novel in situ deposition of hydroxyapatite nanoplates using anodization/hydrothermal process onto magnesium alloy surface towards third generation biomaterials. Mater. Lett. 2016, 164, 144–147. [Google Scholar] [CrossRef]
- Lee, E.; Kwak, D.-H.; Kim, D. Mechanical properties of cellulose acetate/hydroxyapatite nanoparticle composite fiber by electro-spinning process. J. Ceram. Process. Res. 2015, 16, 330–334. [Google Scholar]
- Shaban, N.Z.; Kenawy, M.Y.; Taha, N.A.; El-latif, M.M.A.; Ghareeb, D.A. Synthesized Nanorods Hydroxyapatite by Microwave-Assisted Technology for In Vitro Osteoporotic Bone Regeneration through Wnt/β -Catenin Pathway. Materials 2021, 14, 5823. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Perumalla, S.R.; Lu, R.; Fang, J.; Sun, C.C. Sweet Berberine. Cryst. Growth Des. 2016, 16, 933–939. [Google Scholar] [CrossRef]
- Zhou, L.; Song, F.; Liu, Q.; Yang, M.; Zhao, J.; Tan, R.; Xu, J.; Zhang, G.; Quinn, J.M.W.; Tickner, J.; et al. Berberine sulfate attenuates osteoclast differentiation through RANKL induced NF-κB and NFAT pathways. Int. J. Mol. Sci. 2015, 16, 27087–27096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaban, N.Z.; Talaat, I.M.; Elrashidy, F.H.; Hegazy, A.Y.; Sultan, A.S. Therapeutic role of Punica granatum (pomegranate) seed oil extract on bone turnover and resorption induced in ovariectomized rats. J. Nutr. Heal. Aging 2017, 21, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, J.; Zhang, W. Berberine for bone regeneration: Therapeutic potential and molecular mechanisms. J. Ethnopharmacol. 2021, 277, 114249. [Google Scholar] [CrossRef]
- Samadian, H.; Zamiri, S.; Ehterami, A.; Farzamfar, S.; Vaez, A.; Khastar, H.; Alam, M.; Ai, A.; Derakhshankhah, H.; Allahyari, Z.; et al. Electrospun cellulose acetate/gelatin nanofibrous wound dressing containing berberine for diabetic foot ulcer healing: In vitro and in vivo studies. Sci. Rep. 2020, 10, 8312. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, D.A.; El-Rashidy, F.H.; El-Mallawany, S. Imbalanced diet deficient in calcium and vitamin D- Induced juvenile osteopenia in rats; the potential therapeutic effect of Egyptian Moghat roots water extract (glossostemon bruguieri). Iran. J. Pharm. Res. 2014, 13, 623–634. [Google Scholar] [PubMed]
- Meselhy, M.R. Constituents from Moghat, the roots of Glossostemon bruguieri (Desf.). Molecules 2003, 8, 614–621. [Google Scholar] [CrossRef] [Green Version]
- Munir, M.W. Classification of Electrospinning Methods; Dhara, U.A.E.-M.S.G.E.-S., Ed.; IntechOpen: Rijeka, Croatia, 2020; Chapter 13; ISBN 978-1-78984-469-6. [Google Scholar]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 2020, 12, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, N.; Jiang, C.; Uzunalli, G.; Thankappan, S.K.; Laurencin, C.T.; Deng, M. Polymeric Electrospinning for Musculoskeletal Regenerative Engineering. Regen. Eng. Transl. Med. 2016, 2, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Ao, C.; Niu, Y.; Zhang, X.; He, X.; Zhang, W.; Lu, C. Fabrication and characterization of electrospun cellulose/nano-hydroxyapatite nanofibers for bone tissue engineering. Int. J. Biol. Macromol. 2017, 97, 568–573. [Google Scholar] [CrossRef]
- Cindradewi, A.W.; Bandi, R.; Park, C.W.; Park, J.S.; Lee, E.A.; Kim, J.K.; Kwon, G.J.; Han, S.Y.; Lee, S.H. Preparation and characterization of cellulose acetate film reinforced with cellulose nanofibril. Polymers 2021, 13, 2990. [Google Scholar] [CrossRef] [PubMed]
- Sofi, H.S.; Akram, T.; Shabir, N.; Vasita, R.; Jadhav, A.H. Regenerated cellulose nanofibers from cellulose acetate: Incorporating hydroxyapatite (HAp) and silver (Ag) nanoparticles (NPs), as a scaffold for tissue engineering applications. Mater. Sci. Eng. C 2021, 118, 111547. [Google Scholar] [CrossRef]
- Shalan, A.E.; Afifi, M.; El-Desoky, M.M.; Ahmed, M.K. Electrospun nanofibrous membranes of cellulose acetate containing hydroxyapatite co-doped with Ag/Fe: Morphological features, antibacterial activity and degradation of methylene blue in aqueous solution. New J. Chem. 2021, 45, 9212–9220. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Recent advances in applications of cellulose derivatives-based composite membranes with hydroxyapatite. Materials 2020, 13, 2481. [Google Scholar] [CrossRef]
- ASTM D 882-02 Standard Test Method for Tensile Properties of Thin Plastic Sheeting, ASTM International. Available online: www.Astm.Org (accessed on 10 October 2021).
- Abdolzadeh, H.; Doosthoseini, K. Evaluation of old corrugated container and wood fiber application on surface roughness of three-layer particleboard. BioResources 2009, 4, 970–978. [Google Scholar]
- Journal, A.I.; Samadian, H.; Salehi, M.; Farzamfar, S.; Vaez, A.; Sahrapeyma, H.; Goodarzi, A.; Ghorbani, S. In vitro and in vivo evaluation of electrospun cellulose acetate/gelatin/hydroxyapatite nanocomposite mats for wound dressing applications. Artif. Cells Nanomed. Biotechnol. 2018, 1–11. [Google Scholar]
- Grabska-Zielińska, S.; Sionkowska, A.; Reczyńska, K.; Pamuła, E. Physico-Chemical Characterization and Biological Tests of Collagen/Silk Fibroin/Chitosan Scaffolds Cross-Linked by Dialdehyde Starch. Polymers 2020, 12, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goetz, L.A.; Naseri, N.; Nair, S.S.; Karim, Z.; Mathew, A.P. All cellulose electrospun water purification membranes nanotextured using cellulose nanocrystals. Cellulose 2018, 25, 3011–3023. [Google Scholar] [CrossRef] [Green Version]
- Mikaeili, F.; Gouma, P.I. Super Water-Repellent Cellulose Acetate Mats. Sci. Rep. 2018, 8, 1–8. [Google Scholar]
- Zarbayjani, A.F.A.; Han, S.Y.C. Single and Multi-Layered Nanofibers for Rapid and Controlled Drug Delivery. Chem. Pharm. Bull. 2010, 58, 143–146. [Google Scholar] [CrossRef] [Green Version]
- Suwantong, O.; Opanasopit, P.; Ruktanonchai, U.; Supaphol, P. Electrospun cellulose acetate fiber mats containing curcumin and release characteristic of the herbal substance. Polymer 2007, 48, 7546–7557. [Google Scholar] [CrossRef]
- Nakielski, P.; Kowalczyk, T.; Kowalewski, T.A. Drug delivery system based on polymer nano- fibers. IPPT Rep. Fundam. Technol. Res. 2013, 4, 19–24. [Google Scholar]
- Al-Faris, N.A. Nutritional and Safety Evaluation of Local Weight-Gain Formulas in the Kingdom of Saudi Arabia (KSA) Markets. Food Nutr. Sci. 2014, 5, 1341–1351. [Google Scholar] [CrossRef] [Green Version]
- Łopianiak, I.; Butruk-Raszeja, B.A. Evaluation of sterilization/disinfection methods of fibrous polyurethane scaffolds designed for tissue engineering applications. Int. J. Mol. Sci. 2020, 21, 8092. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Huang, W.; Yang, S.; Shao, J.; Li, Y.P. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front. Biosci. 2007, 12, 3068–3092. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Sample Code | Cellulose Acetate Concentration (w%) | HA Concertation (mg) | HA/BER Composite Concertation (mg) | HA/ME Composite Concentration (mg) | Feeding Rate (mL/h) | Applied Voltage (KV) | Distance from Needle to Collector (cm) | Fiber Morphology |
|---|---|---|---|---|---|---|---|---|
| CA10 | 10 | / | / | / | 0.5 | 24 | 15 | Beaded (Figure S1) |
| CA11 | 11 | / | / | / | 0.5 | 24 | 15 | Beaded (Figure S1) |
| CA12 | 12 | / | / | / | 0.5 | 24 | 15 | Beaded (Figure S1) |
| CA13 | 13 | / | / | / | 0.5 | 24 | 15 | Unbeaded (Figure 1) |
| CA14 | 14 | / | / | / | 0.5 | 24 | 15 | Beaded (Figure S1) |
| CA15 | 15 | / | / | / | 0.5 | 24 | 15 | Unbeaded (Figure S1) |
| CA13/HA25 | 13 | 25 | / | / | 0.5 | 26 | 15 | Unbeaded (Figure S2) |
| CA13/HA50 | 13 | 50 | / | / | 0.5 | 26 | 15 | Unbeaded (Figure 1) |
| CA13/HA100 | 13 | 100 | / | / | 0.5 | 26 | 15 | Beaded (Figure S2) |
| CA13/HA200 | 13 | 200 | / | / | 0.5 | 26 | 15 | Precipitated (Figure S2) |
| CA13/HA/BER25 | 13 | / | 25 | / | 0.5 | 26 | 15 | Unbeaded (Figure S3) |
| CA13/HA/BER50 | 13 | / | 50 | / | 0.5 | 27 | 15 | Unbeaded (Figure S3) |
| CA13/HA/BER100 | 13 | / | 100 | / | 0.5 | 27 | 15 | Unbeaded (Figure 1) |
| CA13/HA/BER200 | 13 | / | 200 | / | 0.5 | 27 | 15 | Beaded (Figure S3) |
| CA13/HA/ME25 | 13 | / | / | 25 | 0.5 | 30 | 15 | Unbeaded (Figure S4) |
| CA13/HA/ME50 | 13 | / | / | 50 | 0.5 | 30 | 15 | Unbeaded (Figure S4) |
| CA13/HA/ME100 | 13 | / | / | 100 | 0.5 | 30 | 15 | Unbeaded (Figure 1) |
| CA13/HA/ME200 | 13 | / | / | 200 | 0.5 | 30 | 15 | Beaded (Figure S4) |
| Gene Name/Base Pair | Primer Sequence | Annealing Temperature (°C) | Number of Cycles | |
|---|---|---|---|---|
| GAPDH | F | 5′-AGATCCACAACGGATACATT-3′ | 52 | 35 |
| R | 5′-TCCCTCAAGATTGTCAGCAA-3′ | |||
| DKK1 | F | 5′-GCTGCATGAGGCACGCTAT-3′ | 55 | 35 |
| R | 5′-AGGGCATGCATATTCCGTTT-3′ | |||
| SOST | F | 5′-GTGCAAGTGCAAGCGCCTCA-3′ | 60 | 40 |
| R | 5′-GCTCCGCCTGGTTGGCTTTG-3′ | |||
| Sox9 | F | 5′-TCCAGCAAGAACAAGCCACA-3′ | 56 | 40 |
| R | 5′-CGAAGGGTCTCTTCTCGCTC-3′ | |||
| RUNX2 | F | 5′-AGTGTGTGTGTCCGCATGAT-3′ | 56 | 40 |
| R | 5′-CCACTTGGGGTCTAAGAACG-3′ | |||
| Osterix | F | 5′-TGAGGAAGAAGCCCATTCAC-3′ | 53.5 | 40 |
| R | 5′-ACTTCTTCTCCCGGGTGTG-3′ | |||
| COLA1 | F | 5′-CAAGGACTATGAAGTTGATGC-3′ | 43 | 40 |
| R | 5′-ACCAGTAGAGAAATCGCAGT-3′ | |||
| OPG | F | 5′-GTTCTTGCACAGCTTCACCA-3′ | 54 | 40 |
| R | 5′-AAACAGCCCAGTGACCATTC-3′ | |||
| RANKL | F | 5′-ACCAGCATCAAAATCCCAAG-3′ | 52 | 35 |
| R | 5′-GGCCGCTAATTTCCTCACCA-3′ | |||
| Samples | Shear Viscosity (Pa·s) | Thickness (mm) | Surface Roughness Ra (µm) | Tensile Strength (MPa) | Maximum Strain (%) |
|---|---|---|---|---|---|
| CA13 | 2.399 ± 0.32 | 0.20 ± 0.04 | 0.11 ± 0.04 | 0.456 ± 0.23 | 2.160 ± 0.01 |
| CA13/HA50 | 1.899 ± 0.34 | 0.24 ± 0.04 | 0.93 ± 0.51 | 1.016 ± 0.25 | 11.340 ± 0.02 |
| CA13/HA/BER100 | 0.844 ± 0.50 | 0.22 ± 0.23 | 0.41 ± 0.23 | 1.278 ± 0.52 | 5.472 ± 0.01 |
| CA13/HA/ME100 | 1.837 ± 0.35 | 0.21 ± 0.79 | 0.63 ± 0.79 | 1.302 ± 0.34 | 5.150 ± 0.01 |
| Samples | Contact Angle (°) | Image | Surface Energy (mN/m) | Porosity (%) | Water Uptake (%) |
|---|---|---|---|---|---|
| CA13 | 113.46 ± 1.46 |  | 20.01 ± 0.86 | 0.89 ± 0.04 | 13.67 ± 0.65 |
| CA13/HA50 | 53.46 ± 0.58 |  | 53.76 ± 0.96 | 0.98 ± 0.51 | 26.20 ± 0.46 |
| CA13/HA/BER100 | 56.46 ± 0.56 |  | 56.66 ± 0.92 | 0.99 ± 0.23 | 25.62 ± 0.56 |
| CA13/HA/ME100 | 103.46 ± 1.04 |  | 20.45 ± 0.86 | 1.01 ± 0.79 | 26.18 ± 0.58 |
| Groups | TRAcP (µg/mL) | Calcium Concentration (mg/mL) | Total Protein Concentration (mg/mL) | Cell Viability (%) | |
|---|---|---|---|---|---|
| Control osteoblast cells (OB) | 0.28 ± 0.06 a | 0.46 ± 0.09 b | 11.36 ± 0.90 c | 100.10 ± 5.19 c | |
| OB | CA13 | 0.33 ± 0.05 ab | 0.92 ± 0.05 c | 5.14 ± 0.73 a | 86.60 ± 3.73 b |
| OB | CA13/HA50 | 0.41 ± 0.05 b | 0.98 ± 0.07 c | 16.02 ± 1.11 d | 146.00 ± 2.69 d |
| OB | CA13/HA/BER100 | 0.39 ± 0.09 b | 0.97 ± 0.05 c | 9.79 ± 0.47 b | 100.01 ± 1.65 c |
| OB | CA13/HA/ME100 | 0.45 ± 0.06 b | 0.98 ± 0.07 c | 11.93 ± 0.45 c | 102.40 ± 2.79 c |
| Induction osteoclast cells (OC) | 1.86 ± 0.04 g | 0.21 ± 0.06 a | 9.07 ± 0.76 b | 75.03 ± 5.79 a | |
| OC | CA13 | 1.23 ± 0.07 d | 0.95 ± 0.04 c | 5.21 ± 0.88 a | 79.40 ± 2.59 a |
| OC | CA13/HA50 | 1.05 ± 0.09 c | 0.90 ± 0.06 c | 4.85 ± 1.49 a | 76.77 ± 2.77 a |
| OC | CA13/HA/BER100 | 1.47 ± 0.05 f | 0.94 ± 0.05 c | 5.57 ± 0.70 a | 86.85 ± 1.08 b |
| OC | CA13/HA/ME100 | 1.35 ± 0.06 e | 0.96 ± 0.03 c | 6.43 ± 0.84 a | 83.75 ± 1.16 b |
| Groups | Osteocalcin (ng/mL) | ALP Activity (%) | CD90 (ng/mg Protein) | P38-MAPK (ng/mg Protein) | PARP- ɣ (pg/mg Protein) | |
|---|---|---|---|---|---|---|
| Control osteoblast cells (OB) | 1.21 ± 0.03 e | 21.82 ± 1.27 e | 2.21 ± 0.03 c | 0.43 ± 0.03 a | 11.44 ± 0.3 a | |
| OB | CA 13 | 1.53 ± 0.05 f | 16.63 ± 0.82 d | 2.01 ± 0.12 a | 0.42 ± 0.02 a | 12.23 ± 0.6 a |
| OB | CA13/HA50 | 2.14 ± 0.04 h | 27.27 ± 1.21 g | 2.23 ± 0.11 c | 0.44 ± 0.03 a | 15.31 ± 0.8 b |
| OB | CA13/HA/BER100 | 1.78 ± 0.07 g | 21.82 ± 1.83 e | 2.12 ± 0.12 b | 0.40 ± 0.04 a | 13.62 ± 0.8 ab |
| OB | CA13/HA/ME100 | 1.69 ± 0.07 g | 24.54 ± 0.77 f | 2.21 ± 0.03 c | 0.57 ± 0.02 b | 25.13 ± 0.6 c |
| Induction osteoclast cells (OC) | 0.53 ± 0.08 a | 05.45 ± 1.08 a | 6.32 ± 0.28 g | 2.53 ± 0.08 g | 39.24 ± 0.6 f | |
| OC | CA 13% | 0.67 ± 0.05 b | 06.36 ± 1.54 a | 4.92 ± 0.19 f | 2.43 ± 0.12 f | 35.83 ± 0.7 f |
| OC | CA13/HA50 | 0.84 ± 0.07 c | 10.91 ± 1.64 b | 3.98 ± 0.25 e | 1.73 ± 0.25 e | 13.64 ± 0.7 ab |
| OC | CA13/HA/BER100 | 0.98 ± 0.07 d | 13.63 ± 0.90 c | 2.94 ± 0.17 d | 1.45 ± 0.23 c | 15.36 ± 0.6 b |
| OC | CA13/HA/ME100 | 0.86 ± 0.07 c | 19.08 ± 1.55 e | 3.08 ± 0.17 d | 1.57 ± 0.22 d | 13.73 ± 0.3 ab |
| Groups | Wnt-5 (ng/mg Protein) | GSK3βpS9 (µg/mg Protein) | GSK3β (µg/mg Protein) | GSK3βpS9/GSK3β | β.Catenin (pg/mg Protein) | |
|---|---|---|---|---|---|---|
| Control osteoblast cells (OB) | 2.48 ± 0.23 b | 4.5 ± 0.23 a | 6.8 ± 0.32 a | 0.66 | 1.45 ± 0.005 c | |
| OB | CA 13 | 2.58 ± 0.25 b | 4.4 ± 0.21 a | 6.3 ± 0.22 a | 0.69 | 1.32 ± 0.003 a |
| OB | CA13/HA50 | 2.85 ± 0.26 c | 4.5 ± 0.11 a | 6.7 ± 0.23 a | 0.67 | 1.42 ± 0.001 bc |
| OB | CA13/HA/BER100 | 2.73 ± 0.23 c | 3.9 ± 0.23 a | 6.8 ± 0.19 a | 0.57 | 1.37 ± 0.007 b |
| OB | CA13/HA/ME100 | 2.17 ± 0.22 a | 3.7 ± 0.21 a | 6.1 ± 0.23 a | 0.61 | 1.46 ± 0.003 c |
| Induction osteoclast cells (OC) | 7.85 ± 0.32 g | 16.2 ± 0.89 e | 15.3 ± 0.89 e | 1.06 | 3.97 ± 0.023 g | |
| OC | CA 13% | 5.93 ± 0.23 f | 9.8 ± 1.50 d | 13.0 ± 0.77 d | 0.75 | 3.63 ± 0.015 f |
| OC | CA13/HA50 | 5.17 ± 0.25 e | 9.0 ± 0.97 d | 10.9 ± 0.72 c | 0.82 | 2.87 ± 0.009 e |
| OC | CA13/HA/BER100 | 3.73 ± 0.29 d | 7.5 ± 1.35 b | 7.6 ± 0.93 b | 0.99 | 1.98 ± 0.008 d |
| OC | CA13/HA/ME100 | 4.01 ± 0.10 e | 8.8 ± 1.26 bc | 10.0 ± 0.45 c | 0.88 | 2.12 ± 0.003 d |
| Groups | Osterix | Runx2 | Cola1 | |
|---|---|---|---|---|
| Control osteoblast cells (OB) | 1 ± 0.0000 e | 1 ± 0.0000 e | 1 ± 0.0000 e | |
| OB | CA 13 | 1 ± 0.0020 e | 1 ± 0.0015 e | 1 ± 0.0023 e |
| OB | CA13/HA50 | 1.02 ± 0.11 e | 1.02 ± 0.02 e | 1.03 ± 0.03 e |
| OB | CA13/HA/BER100 | 1.12 ± 0.03 e | 1.05 ± 0.01 e | 1.09 ± 0.02 f |
| OB | CA13/HA/ME100 | 1.05 ± 0.01 e | 1.04 ± 0.03 e | 1.06 ± 0.02 e |
| Induction osteoclast cells (OC) | 0.15 ± 0.03 a | 0.27 ± 0.01 a | 0.11 ± 0.01 a | |
| OC | CA 13 | 0.23 ± 0.01 b | 0.32 ± 0.03 b | 0.13 ± 0.03 a |
| OC | CA13/HA50 | 0.63 ± 0.01 c | 0.89 ± 0.02 c | 0.59 ± 0.04 b |
| OC | CA13/HA/BER100 | 0.98 ± 0.01 d | 0.99 ± 0.01 e | 0.83 ± 0.03 d |
| OC | CA13/HA/ME100 | 0.97 ± 0.01 d | 0.93 ± 0.03 d | 0.78 ± 0.07 c |
| Groups | SOST | DKK | SOX9 | |
|---|---|---|---|---|
| Control osteoblast cells (OB) | 1 ± 0.0000 a | 1 ± 0.00000 a | 1 ± 0.0000 a | |
| OB | CA 13 | 0.98 ± 0.01 a | 1.08 ± 0.022 b | 1 ± 0.0016 a |
| OB | CA13/HA50 | 1.05 ± 0.02 b | 1.06 ± 0.011 b | 1.01 ± 0.03 a |
| OB | CA13/HA/BER100 | 1.12 ± 0.01 b | 1.03 ± 0.032 b | 1.07 ± 0.02 b |
| OB | CA13/HA/ME100 | 1.08 ± 0.03 b | 1.08 ± 0.023 b | 1.03 ± 0.01 a |
| Induction osteoclast cells (OC) | 6.87 ± 0.03 g | 12.25 ± 0.01 g | 2.93 ± 0.05 f | |
| OC | CA 13 | 5.12 ± 0.01 f | 9.36 ± 0.031 f | 2.81 ± 0.07 e |
| OC | CA13/HA50 | 4.23 ± 0.03 e | 7.23 ± 0.032 e | 2.15 ± 0.03 d |
| OC | CA13/HA/BER100 | 3.96 ± 0.07 d | 5.87 ± 0.042 d | 1.56 ± 0.07 c |
| OC | CA13/HA/ME100 | 2.29 ± 0.03 c | 4.26 ± 0.013 c | 1.63 ± 0.03 c |
| Groups | RANKL | OPG | RANKL/OPG | |
|---|---|---|---|---|
| Control osteoblast cells (OB) | 1 ± 0.0000 a | 1 ± 0.0000 d | 1 ± 0.0000 a | |
| OB | CA 13 | 1.03 ± 0.02 a | 0.99 ± 0.03 d | 1.13 ± 0.01 b |
| OB | CA13/HA50 | 0.98 ± 0.01 a | 0.98 ± 0.02 d | 1.42 ± 0.04 d |
| OB | CA13/HA/BER100 | 1.08 ± 0.03 a | 1.32 ± 0.01 e | 1.22 ± 0.03 c |
| OB | CA13/HA/ME100 | 1.04 ± 0.02 a | 1.23 ± 0.12 e | 1.15 ± 0.01 b |
| Induction osteoclast cells (OC) | 13.7 ± 0.92 f | 0.41 ± 0.01 a | 23.5 ± 1.32 g | |
| OC | CA 13 | 11.1 ± 1.12 e | 0.45 ± 0.03 a | 21.2 ± 1.17 g |
| OC | CA13/HA50 | 8.32 ± 0.47 d | 0.74 ± 0.04 b | 14.3 ± 1.02 f |
| OC | CA13/HA/BER100 | 3.56 ± 0.08 b | 0.97 ± 0.03 d | 9.38 ± 0.08 e |
| OC | CA13/HA/ME100 | 4.57 ± 0.09 c | 0.83 ± 0.02 c | 10.2 ± 0.03 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaban, N.Z.; Kenawy, M.Y.; Taha, N.A.; Abd El-Latif, M.M.; Ghareeb, D.A. Cellulose Acetate Nanofibers: Incorporating Hydroxyapatite (HA), HA/Berberine or HA/Moghat Composites, as Scaffolds to Enhance In Vitro Osteoporotic Bone Regeneration. Polymers 2021, 13, 4140. https://doi.org/10.3390/polym13234140
Shaban NZ, Kenawy MY, Taha NA, Abd El-Latif MM, Ghareeb DA. Cellulose Acetate Nanofibers: Incorporating Hydroxyapatite (HA), HA/Berberine or HA/Moghat Composites, as Scaffolds to Enhance In Vitro Osteoporotic Bone Regeneration. Polymers. 2021; 13(23):4140. https://doi.org/10.3390/polym13234140
Chicago/Turabian StyleShaban, Nadia Z., Marwa Y. Kenawy, Nahla A. Taha, Mona M. Abd El-Latif, and Doaa A. Ghareeb. 2021. "Cellulose Acetate Nanofibers: Incorporating Hydroxyapatite (HA), HA/Berberine or HA/Moghat Composites, as Scaffolds to Enhance In Vitro Osteoporotic Bone Regeneration" Polymers 13, no. 23: 4140. https://doi.org/10.3390/polym13234140
APA StyleShaban, N. Z., Kenawy, M. Y., Taha, N. A., Abd El-Latif, M. M., & Ghareeb, D. A. (2021). Cellulose Acetate Nanofibers: Incorporating Hydroxyapatite (HA), HA/Berberine or HA/Moghat Composites, as Scaffolds to Enhance In Vitro Osteoporotic Bone Regeneration. Polymers, 13(23), 4140. https://doi.org/10.3390/polym13234140