3-Mercaptopropanoic Acid-Doped Chitosan/Hybrid-Based Multilayer Sol-Gel Coatings for Cu Protection in 3.5% NaCl Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cu Metal Polishing and Synthesis of Sol-Gel and Self-Assembly Coating
2.3. Characterization of Modified/Unmodified Cu Electrodes
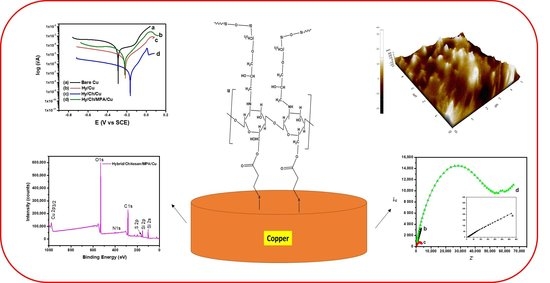
3. Results and Discussion
3.1. Fourier Transform Infrared (FT-IR) Analysis
3.2. X-ray Photoelectron Spectroscopy (XPS) Analysis
3.3. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDX) Analysis
3.4. Atomic Force Microscopy (AFM) Analysis
3.5. Electrochemical Impedance Spectroscopy (EIS)
3.6. Potentiodynamic Polarization Studies (PDS)
3.7. Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bao, Q.; Zhang, D.; Wan, Y. 2-Mercaptobenzothiazole doped chitosan/11-alkanethiolate acid composite coating: Dual function for copper protection. Appl. Surf. Sci. 2011, 257, 10529–10534. [Google Scholar] [CrossRef]
- Rao, B.A.; Iqbal, M.Y.; Sreedhar, B. Self-assembled monolayer of 2-(octadecylthio) benzothiazole for corrosion protection of copper. Corros. Sci. 2009, 5, 1441–1452. [Google Scholar]
- Vera, R.; Bastidas, F.; Villarroel, M.; Oliva, A.; Molinari, A.; Ramírez, D.; del Rio, R. Corrosion inhibition of copper in chloride media by 1, 5-bis (4-dithiocarboxylate-1-dodecyl-5-hydroxy-3-methylpyrazolyl) pentane. Corros. Sci. 2008, 50, 729–736. [Google Scholar] [CrossRef]
- Fonder, G.; Laffineur, F.; Delhalle, J.; Mekhalif, Z. Alkanethiol-oxidized copper interface: The critical influence of concentration. J. Colloid Interface Sci. 2008, 326, 333–338. [Google Scholar] [CrossRef]
- Nurioglu, A.G.; Esteves, A.C. Non-toxic, non-biocide-release antifouling coatings based on molecular structure design for marine applications. J. Mater. Chem. B 2015, 3, 6547–6570. [Google Scholar] [CrossRef] [Green Version]
- Pareek, S.; Jain, D.; Hussain, S.; Biswas, A.; Shrivastava, R.; Parida, S.K.; Kisan, H.K.; Lgaz, H.; Chung, I.M.; Behera, D. A new insight into corrosion inhibition mechanism of copper in aerated 3.5 wt.% NaCl solution by eco-friendly ImidazopyrimidineDye: Experimental and theoretical approach. Chem. Eng. J. 2019, 358, 725–742. [Google Scholar] [CrossRef]
- Hamidon, T.S.; Hussin, M.H. Susceptibility of hybrid sol-gel (TEOS-APTES) doped with caffeine as potent corrosion protective coatings for mild steel in 3.5 wt.% NaCl. Prog. Org. Coat. 2020, 140, 105478. [Google Scholar] [CrossRef]
- Daubert, J.S.; Hill, G.T.; Gotsch, H.N.; Gremaud, A.P.; Ovental, J.S.; Williams, P.S.; Oldham, C.J.; Parsons, G.N. Corrosion protection of copper using Al2O3, TiO2, ZnO, HfO2, and ZrO2 atomic layer deposition. ACS Appl. Mater. Interfaces 2017, 9, 4192–4201. [Google Scholar] [CrossRef] [PubMed]
- Figueira, R.B.; Silva, C.J.; Pereira, E.V. Organic–inorganic hybrid sol–gel coatings for metal corrosion protection: A review of recent progress. J. Coat. Technol. Res. 2015, 12, 1–35. [Google Scholar] [CrossRef]
- Balaji, J.; Roh, S.H.; Edison, T.N.; Jung, H.Y.; Sethuraman, M.G. Sol-gel based hybrid silane coatings for enhanced corrosion protection of copper in aqueous sodium chloride. J. Mol. Liq. 2020, 302, 112551. [Google Scholar] [CrossRef]
- Asadi, N.; Naderi, R.; Saremi, M.; Arman, S.Y.; Fedel, M.; Deflorian, F. Study of corrosion protection of mild steel by eco-friendly silane sol–gel coating. J. Sol Gel Sci. Technol. 2014, 70, 329–338. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Salvado, I.M.; Ferreira, M.G. Sol-gel coatings for corrosion protection of metals. J. Mater. Chem. 2005, 15, 5099–5111. [Google Scholar] [CrossRef]
- Samiee, R.; Ramezanzadeh, B.; Mahdavian, M.; Alibakhshi, E. Assessment of the smart self-healing corrosion protection properties of a water-base hybrid organo-silane film combined with non-toxic organic/inorganic environmentally friendly corrosion inhibitors on mild steel. J. Clean. Prod. 2019, 220, 340–356. [Google Scholar] [CrossRef]
- Kumar, M.N. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Balaji, J.; Sethuraman, M.G. Chitosan-doped-hybrid/TiO2nanocomposite based sol-gel coating for the corrosion resistance of aluminum metal in 3.5% NaCl medium. Int. J. Biol. Macromol. 2017, 104, 1730–1739. [Google Scholar]
- Balaji, J.; Raja, P.B.; Sethuraman, M.G.; Oh, T.H. Experimental and multiscale simulation studies on Chitosan dopedHybrid/Zirconium—A bio-nanocomposite coating for aluminium protection. J. Sol Gel Sci. Technol. 2021, 1–11. [Google Scholar] [CrossRef]
- Ruhi, G.; Modi, O.P.; Dhawan, S.K. Chitosan-polypyrrole-SiO2 composite coatings with advanced anticorrosive properties. Synth. Met. 2015, 200, 24–39. [Google Scholar] [CrossRef]
- Carneiro, J.; Tedim, J.; Fernandes, S.C.; Freire, C.S.; Gandini, A.; Ferreira, M.G.; Zheludkevich, M.L. Functionalized chitosan-based coatings for active corrosion protection. Surf. Coat. Technol. 2013, 226, 51–59. [Google Scholar] [CrossRef]
- Laibinis, P.E.; Whitesides, G.M. Self-assembled monolayers of n-alkanethiolates on copper are barrier films that protect the metal against oxidation by air. J. Am. Chem. Soc. 1992, 114, 9022–9028. [Google Scholar] [CrossRef]
- Álvarez, D.; Collazo, A.; Hernández, M.; Nóvoa, X.R.; Pérez, C. Characterization of hybrid sol–gel coatings doped with hydrotalcite-like compounds to improve corrosion resistance of AA2024-T3 alloys. Prog. Org. Coat. 2010, 68, 91–99. [Google Scholar] [CrossRef]
- Karthik, N.; Sethuraman, M.G. Improved copper corrosion resistance of epoxy-functionalized hybrid sol–gel monolayers by thiosemicarbazide. Ionics 2015, 21, 1477–1488. [Google Scholar] [CrossRef]
- Balgude, D.; Konge, K.; Sabnis, A. Synthesis and characterization of sol–gel derived CNSL based hybrid anti-corrosive coatings. J. Sol Gel Sci. Technol. 2014, 69, 155–165. [Google Scholar] [CrossRef]
- Purcar, V.; Şomoghi, R.; Niţu, S.G.; Nicolae, C.A.; Alexandrescu, E.; Gîfu, I.C.; Gabor, A.R.; Stroescu, H.; Ianchiş, R.; Căprărescu, S.; et al. The effect of different coupling agents on nano-ZnO materials obtained via the sol–gel process. Nanomaterials 2017, 7, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Liu, W.; Wei, Z.; Li, H. Mechanical and thermal properties and morphology of epoxy resins modified by a silicon compound. J. Macromol. Sci. A 2010, 47, 1084–1090. [Google Scholar] [CrossRef]
- Balaji, J.; Sethuraman, M.G. Corrosion protection of copper with hybrid sol-gel containing 1H-1, 2, 4-triazole-3-thiol. Iran. J. Chem. Chem. Eng. 2016, 35, 61–71. [Google Scholar]
- Milošev, I.; Jovanović, Ž.; Bajat, J.B.; Jančić-Heinemann, R.; Mišković-Stanković, V.B. Surface analysis and electrochemical behavior of aluminum pretreated by vinyltriethoxysilane films in mild NaCl solution. J. Electrochem. Soc. 2012, 159, C303. [Google Scholar] [CrossRef]
- Karthik, N.; Asha, S.; Sethuraman, M.G. Influence of pH-sensitive 4-aminothiophenol on the copper corrosion inhibition of hybrid sol–gel monolayers. J. Sol Gel Sci. Technol. 2016, 78, 248–257. [Google Scholar] [CrossRef]
- Ziat, Y.; Abbas, N.; Hammi, M.; Echihi, S. An experimental evaluation of inhibiting corrosion effect of phosphate glass on mild steel in acidic solution. Mater. Res. Express. 2019, 6, 086567. [Google Scholar] [CrossRef]
- Tan, B.; Xiang, B.; Zhang, S.; Qiang, Y.; Xu, L.; Chen, S.; He, J. Papaya leaves extract as a novel eco-friendly corrosion inhibitor for Cu in H2SO4 medium. J. Colloid Interface Sci. 2021, 582, 918–931. [Google Scholar] [CrossRef]
- Tan, B.; He, J.; Zhang, S.; Xu, C.; Chen, S.; Liu, H.; Li, W. Insight into anti-corrosion nature of Betel leaves water extracts as the novel and eco-friendly inhibitors. J. Colloid Interface Sci. 2020, 585, 287–301. [Google Scholar] [CrossRef]
- Fang, J.; Li, J. Quantum chemistry study on the relationship between molecular structure and corrosion inhibition efficiency of amides. J. Mol. Struct. THEOCHEM 2002, 593, 179–185. [Google Scholar] [CrossRef]
- Balaji, J.; Sethuraman, M.G. Corrosion protection of copper with 3-glycidoxypropyltrimethoxysilane-based sol–gel coating through 3-amino-5-mercapto-1, 2, 4-triazole doping. Res. Chem. Intermed. 2016, 42, 1315–1328. [Google Scholar] [CrossRef]
- Innocenzi, P.; Kidchob, T.; Yoko, T. Hybrid organic-inorganic sol-gel materials based on epoxy-amine systems. J. Sol Gel Sci. Technol. 2005, 35, 225–235. [Google Scholar] [CrossRef]














| Nature of Sol-Gel Coating | Average Surface Roughness (Sa) (nm) | Root Mean Square (Sq) (nm) |
|---|---|---|
| Bare Cu | 123.4 | 146.0 |
| Hy/Chitosan/MPA/Cu | 4.6 | 6.2 |
| Nature of Sol-Gel Coatings | −Ecorr (mV) | icorr (µA·cm−2) | ηPDS (%) |
|---|---|---|---|
| Bare Cu | −292 | 7.09 × 10−4 | - |
| Hy/Cu | −222 | 1.18 × 10−4 | 83.3 |
| Hy/Chitosan/Cu | −215 | 7.05 × 10−5 | 90.0 |
| Hy/Chitosan/MPA/Cu | −165 | 4.08 × 10−7 | 99.9 |
| Parameters | Bare Cu | Hy/Cu | Hy/Chitosan/Cu | Hy/Chitosan/MPA/Cu |
|---|---|---|---|---|
| Rs (Ω) | 5.0 | 6.2 | 9.0 | 10.0 |
| Qsam (µF cm−2) | - | 2.25 × 10−5 | 1.01 × 10−5 | 3.11 × 10−7 |
| n1 | - | 0.70 | 0.80 | 0.73 |
| Rsam (Ω cm2) | - | 30.5 | 48.3 | 76.2 |
| Qdl (µF cm−2) | 1.17 × 10−3 | 1.68 × 10−5 | 1.53 × 10−5 | 1.55 × 10−5 |
| n2 | 0.80 | 0.80 | 0.53 | 0.85 |
| Rt (kΩ) | 586.4 | 3557 | 6256 | 9,764,000 |
| η (%) using Rt | - | 83.5 | 90.6 | 99.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balaji, J.; Oh, T.H. 3-Mercaptopropanoic Acid-Doped Chitosan/Hybrid-Based Multilayer Sol-Gel Coatings for Cu Protection in 3.5% NaCl Solution. Polymers 2021, 13, 3743. https://doi.org/10.3390/polym13213743
Balaji J, Oh TH. 3-Mercaptopropanoic Acid-Doped Chitosan/Hybrid-Based Multilayer Sol-Gel Coatings for Cu Protection in 3.5% NaCl Solution. Polymers. 2021; 13(21):3743. https://doi.org/10.3390/polym13213743
Chicago/Turabian StyleBalaji, Jaganathan, and Tae Hwan Oh. 2021. "3-Mercaptopropanoic Acid-Doped Chitosan/Hybrid-Based Multilayer Sol-Gel Coatings for Cu Protection in 3.5% NaCl Solution" Polymers 13, no. 21: 3743. https://doi.org/10.3390/polym13213743
APA StyleBalaji, J., & Oh, T. H. (2021). 3-Mercaptopropanoic Acid-Doped Chitosan/Hybrid-Based Multilayer Sol-Gel Coatings for Cu Protection in 3.5% NaCl Solution. Polymers, 13(21), 3743. https://doi.org/10.3390/polym13213743