Synthesis, Characterization and Structure Properties of Biobased Hybrid Copolymers Consisting of Polydiene and Polypeptide Segments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Synthesis of the Hybrid Material
3. Results and Discussion
3.1. Size Exclusion Chromatography
3.2. Infrared Spectroscopy
3.3. Proton and Carbon Nuclear Magnetic Resonance
3.4. Thermogravimetric Analysis and Differential Scanning Calorimetry
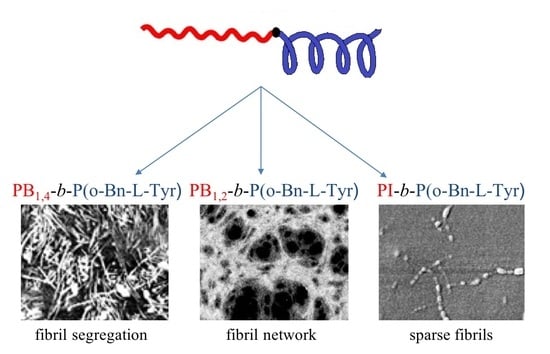
3.5. Atomic Force Microscopy
3.6. Dielectric Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, H.; Gopinadhan, M.; Osuji, C.O. Directed Self-Assembly of Block Copolymers: A Tutorial Review of Strategies for Enabling Nanotechnology with Soft Matter. Soft Matter 2014, 10, 3867–3889. [Google Scholar] [CrossRef]
- Ge, J.; Neofytou, E.; Lei, J.; Beygui, R.E.; Zare, R.N. Protein-Polymer Hybrid Nanoparticles for Drug Delivery. Small 2012, 8, 3573–3578. [Google Scholar] [CrossRef] [PubMed]
- Obermeyer, A.C.; Olsen, B.D. Synthesis and Application of Protein-Containing Block Copolymers. ACS Macro Lett. 2015, 4, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Rosales, A.M.; McCulloch, B.L.; Zuckermann, R.N.; Segalman, R.A. Tunable Phase Behavior of Polystyrene-Polypeptoid Block Copolymers. Macromolecules 2012, 45, 6027–6035. [Google Scholar] [CrossRef]
- Koga, T.; Kamiwatari, S.; Higashi, N. Preparation and Self-Assembly Behavior of β-Sheet Peptide-Inserted Amphiphilic Block Copolymer as a Useful Polymeric Surfactant. Langmuir 2013, 29, 15477–15484. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ferrer, A.; Mezzenga, R. Secondary Structure-Induced Micro- and Macrophase Separation in Rod-Coil Polypeptide Diblock, Triblock and Star-Block Copolymers. Macromolecules 2010, 43, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Tang, P.; Qiu, F.; Yang, Y.; Shi, A.C. Phase Behavior of Semiflexible-Coil Diblock Copolymers: A Hybrid Numerical SCFT Approach. Soft Matter 2011, 7, 929–938. [Google Scholar] [CrossRef]
- Klok, H.A.; Langenwalter, J.F.; Lecommandoux, S. Self-Assembly of Peptide-Based Diblock Oligomers. Macromolecules 2000, 33, 7819–7826. [Google Scholar] [CrossRef]
- Hanski, S.; Houbenov, N.; Ruokolainen, J.; Chondronicola, D.; Iatrou, H.; Hadjichristidis, N.; Ikkala, O. Hierarchical Ionic Self-Assembly of Rod—Comb Block Copolypeptide—Surfactant Complexes. Biomacromolecules 2006, 7, 3379–3384. [Google Scholar] [CrossRef]
- Łosik, M.; Kubowicz, S.; Smarsly, B.; Schlaad, H. Solid-State Structure of Polypeptide-Based Rod-Coil Block Copolymers: Folding of Helices. Eur. Phys. J. E 2004, 15, 407–411. [Google Scholar] [CrossRef]
- Rosales, A.M.; Segalman, R.A.; Zuckermann, R.N. Polypeptoids: A Model System to Study the Effect of Monomer Sequence on Polymer Properties and Self-Assembly. Soft Matter 2013, 9, 8400–8414. [Google Scholar] [CrossRef]
- Lam, C.N.; Olsen, B.D. Phase Transitions in Concentrated Solution Self-Assembly of Globular Protein-Polymer Block Copolymers. Soft Matter 2013, 9, 2393–2402. [Google Scholar] [CrossRef]
- Klok, H.A. Peptide/Protein-Synthetic Polymer Conjugates: Quo Vadis. Macromolecules 2009, 42, 7990–8000. [Google Scholar] [CrossRef]
- Yoda, R.; Hirokawa, Y.; Hayashi, T. Synthesis and Molecular Characterization of A-B-A Type Block Copolymer Consisting of Poly(γ-Benzyl l-Glutamate) as the a Component and Polyisoprene as the B Component. Eur. Polym. J. 1994, 30, 1397–1401. [Google Scholar] [CrossRef]
- Yuan, M.; Deng, X. Synthesis and Characterization of Poly(Ethylene Glycol)-Block-Poly(Amino Acid) Copolymer. Eur. Polym. J. 2001, 37, 1907–1912. [Google Scholar] [CrossRef]
- Janssen, K.; van Beylen, M.; Samyn, C. Morphology of ABA triblock copolymers consisting of poly(γ-benzyl L-glutamate) as the A component and polystyrene as the B component. Makromol. Chem. 1990, 191, 2777–2785. [Google Scholar] [CrossRef]
- Mei, Y.; Beers, K.L.; Byrd, H.C.M.; Van der Hart, D.L.; Washburn, N.R. Solid-Phase ATRP Synthesis of Peptide-Polymer Hybrids. J. Am. Chem. Soc. 2004, 126, 3472–3476. [Google Scholar] [CrossRef]
- Li, T.; Lin, J.; Chen, T.; Zhang, S. Polymeric Micelles Formed by Polypeptide Graft Copolymer and Its Mixtures with Polypeptide Block Copolymer. Polymer 2006, 47, 4485–4489. [Google Scholar] [CrossRef]
- Lin, J.; Zhu, J.; Chen, T.; Lin, S.; Cai, C.; Zhang, L.; Zhuang, Y.; Wang, X.S. Drug Releasing Behavior of Hybrid Micelles Containing Polypeptide Triblock Copolymer. Biomaterials 2009, 30, 108–117. [Google Scholar] [CrossRef]
- Kros, A.; Jesse, W.; Metselaar, G.A.; Cornelissen, J.J.L.M. Synthesis and Self-Assembly of Rod-Rod Hybrid Poly(γ-BenzylL-Glutamate)-Block-Polyisocyanide Copolymers. Angew. Chemie 2005, 117, 4423–4426. [Google Scholar] [CrossRef]
- Hayashi, T.; Chen, G.W.; Nakajima, A. Synthesis and Structural Study of the A-B-A Type Block Copolymer Consisting of Poly(γ-benzyl L-glutamate) as the A Component and Polybutadiene as the B Component. Macromolecules 1979, 12, 840–843. [Google Scholar]
- Kugo, K.; Hayashi, T.; Nakajima, A. Synthesis, Structure, and Mechanical Properties of A-B-A Tri-Block Copolymers Consisting of Poly(e-N-benzyloxycarbonyl-L-lysine) as the A Component andPolybutadiene as the B ComponentStudies on Membrane Surfaces of A-b-a Tri-Block Ccopolymers Consisting of Poly(ε-A-Benzyloxycarbonyl-l-Lysine) as the a Component and Polybutadiene as the Β Component. Polym. J. 1982, 14, 401–410. [Google Scholar]
- Ulyanova, N.N.; Baranovskaya, I.A.; Liubina, S.Y.; Bezrukova, A.; Rudkovskaya, G.D.; Shabsels, B.M.; Vlasov, G.P.; Eskin, V.E. Investigation of Macromolecules Exhibiting the Structure of a Once-Broken Rod by Molecular Optics. 2. Synthesis and Investigation of Three-Block Copolymers: Poly(γ-benzyl L-glutamate)-Poly(methyl methacrylate)-Poly(γ-benzyl L-glutamate). Macromolecules 1991, 24, 3324–3327. [Google Scholar] [CrossRef]
- Ibarboure, E.; Papon, E.; Rodríguez-Hernández, J. Nanostructured Thermotropic PBLG-PDMS-PBLG Block Copolymers. Polymer. 2007, 48, 3717–3725. [Google Scholar] [CrossRef]
- Billot, J.; Douy, A.; Gallot, B. Preparation, Fractionation, and Structure of Block Copolymers Polystyrene-Poly(Carbobenzoxy-L-lysine) and Polybutadiene-Poly(Carbobenzoxy-L-lysine ). Makromol. Chem. 1977, 178, 1641–1650. [Google Scholar] [CrossRef]
- Checot, F.; Lecommandoux, S.; Gnanou, Y.; Klok, H.-A. Water-Soluble Stimuli-Responsive Vesicles from Peptide-Based Diblock Copolymers. Angew. Chem. Int. Ed. 2002, 41, 1339–1343. [Google Scholar] [CrossRef]
- Ayres, L.; Hans, P.; Adams, J.; Löwik, D.W.P.M.; Van Hest, J.C.M. Peptide-Polymer Vesicles Prepared by Atom Transfer Radical Polymerization. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 6355–6366. [Google Scholar] [CrossRef]
- Deming, T.J. Cobalt and Iron Initiators for the Controlled Polymerization of α-Amino Acid-N-Carboxyanhydrides. Macromolecules 1999, 32, 4500–4502. [Google Scholar] [CrossRef]
- Becker, M.L.; Liu, J.; Wooley, K.L. Erratum: Peptide-Polymer Bioconjugates: Hybrid Block Copolymers Generated via Living Radical Polymerizations from Resin-Supported Peptides (Chemical Communications 2003). Chem. Commun. 2003, 1, 802. [Google Scholar]
- Daly, H.W.; Poche, D. The preparation of N-carboxyanhydrides of α-amino acids using bis (trichloromethyl) carbonate. Tetrahedron Lett. 1988, 29, 5859–5862. [Google Scholar] [CrossRef]
- Becker, M.L.; Liu, J.; Wooley, K.L. Functionalized Micellar Assemblies Prepared via Block Copolymers Synthesized by Living Free Radical Polymerization upon Peptide-Loaded Resins. Biomacromolecules 2005, 6, 220–228. [Google Scholar] [CrossRef]
- Broyer, R.M.; Quaker, G.M.; Maynard, H.D. Designed Amino Acid ATRP Initiators for the Synthesis of Biohybrid Materials. J. Am. Chem. Soc. 2008, 130, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Conejos-Sánchez, I.; Duro-Castano, A.; Birke, A.; Barz, M.; Vicent, M.J. A Controlled and Versatile NCA Polymerization Method for the Synthesis of Polypeptides. Polym. Chem. 2013, 4, 3182–3186. [Google Scholar] [CrossRef]
- Junnila, S.; Houbenov, N.; Karatzas, A.; Hadjichristidis, N.; Hirao, A.; Iatrou, H.; Ikkala, O. Side-Chain-Controlled Self-Assembly of Polystyrene-Polypeptide Miktoarm Star Copolymers. Macromolecules 2012, 45, 2850–2856. [Google Scholar] [CrossRef]
- Byrne, M.; Murphy, R.; Kapetanakis, A.; Ramsey, J.; Cryan, S.A.; Heise, A. Star-Shaped Polypeptides: Synthesis and Opportunities for Delivery of Therapeutics. Macromol. Rapid Commun. 2015, 36, 1862–1876. [Google Scholar] [CrossRef]
- Tao, L.; Kaddis, C.S.; Ogorzalek Loo, R.R.; Grover, G.N.; Loo, J.A.; Maynard, H.D. Synthetic Approach to Homodimeric Protein-Polymer Conjugates. Chem. Commun. 2009, 16, 2148–2150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.C.; Kuo, S.W. Hierarchical Self-Assembly Structures of POSS-Containing Polypeptide Block Copolymers Synthesized Using a Combination of ATRP, ROP and Click Chemistry. Polym. Chem. 2012, 3, 882–891. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Iatrou, H.; Pispas, S.; Pitsikalis, M. Anionic polymerization: High vacuum techniques. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 3211–3234. [Google Scholar] [CrossRef]
- Politakos, N.; Weinman, C.J.; Paik, M.Y.; Sundaram, H.S.; Ober, C.K.; Avgeropoulos, A. Synthesis, molecular, and morphological characterization of initial and modified diblock copolymers with organic acid chloride derivatives. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4292–4305. [Google Scholar] [CrossRef]
- Pickel, D.L.; Politakos, N.; Avgeropoulos, A.; Messman, J.M. A Mechanistic Study of α-(Amino acid)-n-carboxyanhydride Polymerization: Comparing Initiation and Termination Events in High-Vacuum and Traditional Polymerization Techniques. Macromolecules 2009, 42, 7781–7788. [Google Scholar] [CrossRef]
- Aliferis, T.; Iatrou, H.; Hadjichristidis, N. Living Polypeptides. Biomacromolecules 2004, 5, 1653–1656. [Google Scholar] [CrossRef]
- Politakos, N.; Liontos, G.; Kortaberria, G.; Messman, J.M.; Calvo, J.; Moya, S.E.; Mays, J.W.; Avgeropoulos, A. Comparing Linear and Cyclic Synthetic Homopolypeptides: Synthesis and Molecular Characterization. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 393–404. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Bioc. Bio. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Avgeropoulos, A.; Paraskeva, S.; Hadjichristidis, N.; Thomas, E.L. Synthesis and Microphase Separation of Linear Triblock Terpolymers Of Polystyrene, High 1,4-Polybutadiene, and High 3,4-Polyisoprene. Macromolecules 2002, 35, 4030–4035. [Google Scholar] [CrossRef]
- Rangou, S.; Moschovas, D.; Moutsios, I.; Manesi, G.; Tsitoni, K.; Bovsunovskaya, P.V.; Ivanov, D.A.; Thomas, E.L.; Avgeropoulos, A. Dendrons and Dendritic Terpolymers: Synthesis, Characterization and Self-Assembly Comparison. Molecules 2020, 25, 6030. [Google Scholar] [CrossRef] [PubMed]
- Mondeshki, M.; Spiess, H.W.; Aliferis, T.; Iatrou, H.; Hadjichristidis, N.; Floudas, G. Hierarchical Self-Assembly in Diblock Copolypeptides of poly(o-benzyl-L-glutamate) with poly(L-leucine) and poly(O-benzyl-L-tyrosine). Eur. Polym. J. 2011, 47, 668–674. [Google Scholar] [CrossRef]
- Douy, A.; Gallot, B. Block Copolymers with a Polyvinyl and a Polypeptide Block: Factors Governing the Folding of the Polypeptide Chains. Polymer 1982, 23, 1039–1044. [Google Scholar] [CrossRef]
- Wang, Y.; Jardetzky, O. Probability-Based Protein Secondary Structure Identification Using Combined NMR Chemical-Shift Data. Protein Sci. 2002, 11, 852–861. [Google Scholar] [CrossRef]
- Moschovas, D.; Manesi, G.; Karydis-Messinis, A.; Zapsas, G.; Ntetsikas, K.; Zafeiropoulos, N.E.; Piryazev, A.A.; Thomas, E.L.; Hadjichristidis, N.; Ivanov, D.A.; et al. Alternating Gyroid Network Structure in an ABC Miktoarm Terpolymer Comprised of Polystyrene and Two Polydienes. Nanomaterials 2020, 10, 1497. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Li, B.; Ren, S. Glass Transition Temperature and Chain Flexibility of 1,2-Polybutadiene. J. Appl. Polym. Sci. 1986, 31, 873–884. [Google Scholar] [CrossRef]
- Chang, C.C.; Halasa, A.F.; Miller, J.W. The Reaction Engineering of the Anionic Polymerization of Isoprene. J. Appl. Polym. Sci. 1993, 47, 1589–1599. [Google Scholar] [CrossRef]
- Widmaier, J.M.; Meyer, G.C. Glass Transition Temperature of Anionic Polyisoprene. Macromolecules 1981, 14, 450–453. [Google Scholar] [CrossRef]
- Van Hest, J.C.M. Biosynthetic-Synthetic Polymer Conjugates. J. Macromol. Sci. Part C Polym. Rev. 2007, 47, 63–92. [Google Scholar] [CrossRef]
- König, H.M.; Kilbinger, A.F.M. Learning from Nature: B -Sheet-Mimicking Copolymers Get Organized. Angew. Chem. Int. Ed. 2007, 46, 8334–8340. [Google Scholar] [CrossRef] [PubMed]
- Del Mercato, L.L.; Pompa, P.P.; Maruccio, G.; Della Torre, A.; Sabella, S.; Tamburro, A.M.; Cingolani, R.; Rinaldi, R. Charge transport and intrinsic fluorescence in amyloid-like fibrils. Proc. Natl. Acad. Sci. USA 2007, 104, 18019–18024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshioka, A.; Komuro, K.; Ueda, A.; Watanabe, H.; Akita, S.; Masuda, T.; Nakajima, A. Structure and Physical Properties of High-Vinyl Polybutadiene Rubbers and Their Blends. Pure Appl. Chem. 1986, 58, 1697–1706. [Google Scholar] [CrossRef] [Green Version]
- Quan, X.; Johnson, G.E.; Anderson, E.W.; Bates, F.S. Block Copolymers near the Microphase Separation Transition. 4. Dielectric Spectroscopy. Macromolecules 1989, 22, 2451–2456. [Google Scholar] [CrossRef]








| Sample | Code Name | Polydiene (SEC) a g/mol | Polypeptide (SEC) a g/mol | (SEC) a g/mol | Đtotal | f (%) Polypeptide |
|---|---|---|---|---|---|---|
| PI1,4-b-P(o-Bn-L-Tyr) | ITnp | 14.300 | 41.500 | 55.800 | 1.10 | 74 |
| PI3,4/1,2-b-P(o-Bn-L-Tyr) | Itp | 16.400 | 44.200 | 60.600 | 1.08 | 73 |
| PB1,4-b-P(o-Bn-L-Tyr) | BTp | 15.400 | 59.500 | 74.900 | 1.09 | 79 |
| PB1,2-b-P(o-Bn-L-Tyr) | BTnp | 22.100 | 83.600 | 105.700 | 1.09 | 79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Politakos, N.; Moutsios, I.; Manesi, G.-M.; Moschovas, D.; Abukaev, A.F.; Nikitina, E.A.; Kortaberria, G.; Ivanov, D.A.; Avgeropoulos, A. Synthesis, Characterization and Structure Properties of Biobased Hybrid Copolymers Consisting of Polydiene and Polypeptide Segments. Polymers 2021, 13, 3818. https://doi.org/10.3390/polym13213818
Politakos N, Moutsios I, Manesi G-M, Moschovas D, Abukaev AF, Nikitina EA, Kortaberria G, Ivanov DA, Avgeropoulos A. Synthesis, Characterization and Structure Properties of Biobased Hybrid Copolymers Consisting of Polydiene and Polypeptide Segments. Polymers. 2021; 13(21):3818. https://doi.org/10.3390/polym13213818
Chicago/Turabian StylePolitakos, Nikolaos, Ioannis Moutsios, Gkreti-Maria Manesi, Dimitrios Moschovas, Ainur F. Abukaev, Evgeniia A. Nikitina, Galder Kortaberria, Dimitri A. Ivanov, and Apostolos Avgeropoulos. 2021. "Synthesis, Characterization and Structure Properties of Biobased Hybrid Copolymers Consisting of Polydiene and Polypeptide Segments" Polymers 13, no. 21: 3818. https://doi.org/10.3390/polym13213818
APA StylePolitakos, N., Moutsios, I., Manesi, G. -M., Moschovas, D., Abukaev, A. F., Nikitina, E. A., Kortaberria, G., Ivanov, D. A., & Avgeropoulos, A. (2021). Synthesis, Characterization and Structure Properties of Biobased Hybrid Copolymers Consisting of Polydiene and Polypeptide Segments. Polymers, 13(21), 3818. https://doi.org/10.3390/polym13213818