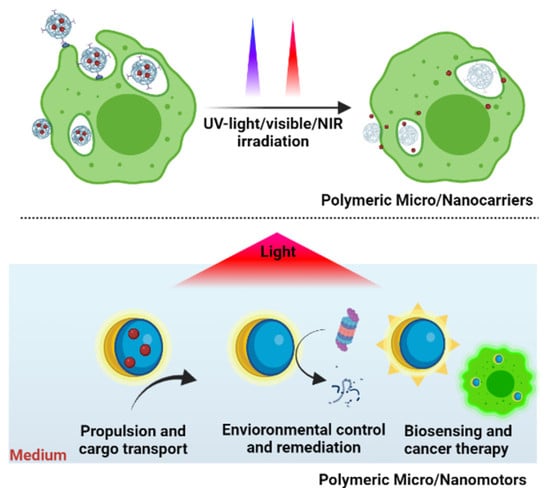
Polymeric Micro/Nanocarriers and Motors for Cargo Transport and Phototriggered Delivery
Abstract
:1. Introduction
2. Organic and Polymeric Micro/Nanocarriers
2.1. Liposomes
2.2. Polymeric Micro/Nanomicelles
2.3. Micro/Nanopolymersomes
2.4. Polymeric Micro/Nanospheres
3. Photosensitive Polymeric Micro/Nanocarriers
3.1. Photoisomerizable and Photocleavable Molecues
3.2. Photoactive Polymers: Synthesis and Assembly
3.3. Photostimulation Mechanisms for Cargo Delivery
3.4. Characterization of Photosensitive Micro/Nanocarriers
3.4.1. Cargo Loading Capacity and Encapsulation Efficiency
3.4.2. Cargo Photorelease
4. Photosensitive Polymeric Nanomicelles
5. Photo-Stimulated Organic and Polymeric Nanocarriers for Cargo Delivery
5.1. Photosensitive Liposomes and Polymeric Nanoconjugates
5.2. Photosensitive Nanopolymersomes
6. Functionalization of Phototriggered Nanocarriers for Targeting Cells

7. Polymeric Micro/Nanomotors for Cargo Transport
7.1. Definition and Classification
7.1.1. Propelled by Light
7.1.2. Propelled by Magnetic Fields and Catalytic Reactions

7.1.3. Propelled by Enzymes
7.1.4. Propelled by Electrophoresis, Diffusiophoresis, and the Marangoni Effect
8. Some Applications of Light-Stimulated Polymeric Micro/Nanomotors
8.1. Propulsion, Cargo Transport, and Drug Delivery

8.2. Environmental Control and Remediation
8.3. Biosensing and Cancer Therapeutics
9. Current Challenges, Opportunities, and Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velasco, V.; Shariati, S.A.; Esfandyarpour, R. Microtechnology-Based Methods for Organoid Models. Microsyst. Nanoeng. 2020, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tay, A.; McCausland, H.; Komeili, A.; di Carlo, D. Nano and Microtechnologies for the Study of Magnetotactic Bacteria. Adv. Funct. Mater. 2019, 29, 1904178. [Google Scholar] [CrossRef] [Green Version]
- Shanmugapriya, K.; Kang, H.W. Engineering Pharmaceutical Nanocarriers for Photodynamic Therapy on Wound Healing. Mater. Sci. Eng. C 2019, 105, 110110. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Y.; Lian, J.; Shen, Q.; Wang, C.; Ma, B.; Zhang, Y.; Xu, T.; Li, J.; Shao, Y. Engineering the Surface of Smart Nanocarriers Using a PH-/Thermal-/GSH-responsive Polymer Zipper for Precise Tumor Targeting Therapy in Vivo. Adv. Mater. 2017, 29, 1702311. [Google Scholar] [CrossRef]
- Jeevarathinam, A.S.; Lemaster, J.E.; Chen, F.; Zhao, E.; Jokerst, J.V. Photoacoustic Imaging Quantifies Drug Release from Nanocarriers via Redox Chemistry of Dye-Labeled Cargo. Angew. Chem. Int. Ed. 2020, 59, 4678–4683. [Google Scholar] [CrossRef]
- Wang, L.; Marciello, M.; Estévez-Gay, M.; Soto Rodriguez, P.E.D.; Luengo Morato, Y.; Iglesias-Fernández, J.; Huang, X.; Osuna, S.; Filice, M.; Sánchez, S. Enzyme Conformation Influences the Performance of Lipase-powered Nanomotors. Angew. Chem. 2020, 132, 21266–21273. [Google Scholar] [CrossRef]
- Huang, L.; Chen, F.; Lai, Y.; Xu, Z.; Yu, H. Engineering Nanorobots for Tumor-targeting Drug Delivery: From Dynamic Control to Stimuli-responsive Strategy; Weliy: Hoboken, NJ, USA; p. 2021. [CrossRef]
- Robertson, B.; Huang, M.-J.; Chen, J.-X.; Kapral, R. Synthetic Nanomotors: Working Together through Chemistry. Acc. Chem. Res. 2018, 51, 2355–2364. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Ng, D.H.L.; Yang, P.; Yang, W.; Liu, Y. Micromotor-Assisted Highly Efficient Fenton Catalysis by a Laccase/Fe-BTC-NiFe 2 O 4 Nanozyme Hybrid with a 3D Hierarchical Structure. Environ. Sci. Nano 2020, 7, 2573–2583. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, S.; Ge, L.; Wu, W.; Jiang, X. Translatable High Drug Loading Drug Delivery Systems Based on Biocompatible Polymer Nanocarriers. Biomacromolecules 2018, 19, 1732–1745. [Google Scholar] [CrossRef]
- Ou, J.; Liu, K.; Jiang, J.; Wilson, D.A.; Liu, L.; Wang, F.; Wang, S.; Tu, Y.; Peng, F. Micro-/Nanomotors toward Biomedical Applications: The Recent Progress in Biocompatibility. Small 2020, 16, 1906184. [Google Scholar] [CrossRef]
- Alkanawati, M.S.; Wurm, F.R.; Thérien-Aubin, H.; Landfester, K. Large-Scale Preparation of Polymer Nanocarriers by High-Pressure Microfluidization. Macromol. Mater. Eng. 2018, 303, 1700505. [Google Scholar] [CrossRef] [Green Version]
- Novotný, F.; Pumera, M. Nanomotor Tracking Experiments at the Edge of Reproducibility. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Essa, D.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. The Design of Poly (Lactide-Co-Glycolide) Nanocarriers for Medical Applications. Front. Bioeng. Biotechnol. 2020, 8, 48. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Q.; Du, X. Light-Powered Micro/Nanomotors. Micromachines 2018, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Venditti, I. Morphologies and Functionalities of Polymeric Nanocarriers as Chemical Tools for Drug Delivery: A Review. J. King Saud Univ. Sci. 2019, 31, 398–411. [Google Scholar] [CrossRef]
- Zha, F.; Wang, T.; Luo, M.; Guan, J. Tubular Micro/Nanomotors: Propulsion Mechanisms, Fabrication Techniques and Applications. Micromachines 2018, 9, 78. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Lv, Y.; Xu, Y.; Zhang, P.; Li, H.; Chen, X.; Li, X.; Zhang, L.; Liu, F.; Zeng, W. Construction of a Biodegradable, Versatile Nanocarrier for Optional Combination Cancer Therapy. Acta Biomater. 2019, 83, 359–371. [Google Scholar] [CrossRef]
- Liu, L.; Gao, J.; Wilson, D.A.; Tu, Y.; Peng, F. Fuel-Free Micro-/Nanomotors as Intelligent Therapeutic Agents. Chem. –Asian J. 2019, 14, 2325–2335. [Google Scholar] [CrossRef]
- El-Sherbiny, I.; Khalil, I.; Ali, I.; Yacoub, M. Updates on Smart Polymeric Carrier Systems for Protein Delivery. Drug Dev. Ind. Pharm. 2017, 43, 1567–1583. [Google Scholar] [CrossRef]
- Li, Y.; Gao, J.; Zhang, C.; Cao, Z.; Cheng, D.; Liu, J.; Shuai, X. Stimuli-Responsive Polymeric Nanocarriers for Efficient Gene Delivery; Springer: Berlin/Heidelberg, Germany, 2017; pp. 167–215. [Google Scholar]
- Efthimiadou, E.K.; Theodosiou, M.; Toniolo, G.; Abu-Thabit, N.Y. Stimuli-Responsive Biopolymer Nanocarriers for Drug Delivery Applications. Stimuli Responsive Polym. Nanocarriers Drug Deliv. Appl. 2018, 1, 405–432. [Google Scholar]
- Rahman, H.S.; Othman, H.H.; Hammadi, N.I.; Yeap, S.K.; Amin, K.M.; Samad, N.A.; Alitheen, N.B. Novel Drug Delivery Systems for Loading of Natural Plant Extracts and Their Biomedical Applications. Int. J. Nanomed. 2020, 15, 2439. [Google Scholar] [CrossRef] [Green Version]
- You, Y.; Xu, D.; Pan, X.; Ma, X. Self-Propelled Enzymatic Nanomotors for Enhancing Synergetic Photodynamic and Starvation Therapy by Self-Accelerated Cascade Reactions. Appl. Mater. Today 2019, 16, 508–517. [Google Scholar] [CrossRef]
- Allen, S.D.; Bobbala, S.; Karabin, N.B.; Scott, E.A. On the Advancement of Polymeric Bicontinuous Nanospheres toward Biomedical Applications. Nanoscale Horiz. 2019, 4, 258–272. [Google Scholar] [CrossRef]
- Shakiba, S.; Astete, C.E.; Paudel, S.; Sabliov, C.M.; Rodrigues, D.F.; Louie, S.M. Emerging Investigator Series: Polymeric Nanocarriers for Agricultural Applications: Synthesis, Characterization, and Environmental and Biological Interactions. Environ. Sci. Nano 2020, 7, 37–67. [Google Scholar] [CrossRef]
- Wang, S.; Liu, K.; Wang, F.; Peng, F.; Tu, Y. The Application of Micro-and Nanomotors in Classified Drug Delivery. Chem. Asian J. 2019, 14, 2336–2347. [Google Scholar] [CrossRef]
- Safdar, M.; Khan, S.U.; Jänis, J. Progress toward Catalytic Micro-and Nanomotors for Biomedical and Environmental Applications. Adv. Mater. 2018, 30, 1703660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, S.J.; Simonelli, A.P.; Higuchi, W.I. Investigation of Factors Influencing Release of Solid Drug Dispersed in Inert Matrices. J. Pharm. Sci. 1965, 54, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Ismagilov, R.F.; Schwartz, A.; Bowden, N.; Whitesides, G.M. Autonomous Movement and Self-assembly. Angew. Chem. Int. Ed. 2002, 41, 652–654. [Google Scholar] [CrossRef]
- Gu, M.; Wang, X.; Toh, T.B.; Chow, E.K.-H. Applications of Stimuli-Responsive Nanoscale Drug Delivery Systems in Translational Research. Drug Discov. Today 2018, 23, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Wang, W. Phototriggered Targeting of Nanocarriers for Drug Delivery. Nano Res. 2018, 11, 5424–5438. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, Y.; Wang, Q.; Liu, T.; Sun, J.; Zhang, R. Remote Light-responsive Nanocarriers for Controlled Drug Delivery: Advances and Perspectives. Small 2019, 15, 1903060. [Google Scholar] [CrossRef]
- Liang, Z.; Tu, Y.; Peng, F. Polymeric Micro/Nanomotors and Their Biomedical Applications; Weliy: Hoboken, NJ, USA, 2021; p. 2100720. [Google Scholar]
- Umapathi, R.; Reddy, P.M.; Rani, A.; Venkatesu, P. Influence of Additives on Thermoresponsive Polymers in Aqueous Media: A Case Study of Poly (N-Isopropylacrylamide). Phys. Chem. Chem. Phys. 2018, 20, 9717–9744. [Google Scholar] [CrossRef]
- Umapathi, R.; Kumar, K.; Rani, G.M.; Venkatesu, P. Influence of Biological Stimuli on the Phase Behaviour of a Biomedical Thermoresponsive Polymer: A Comparative Investigation of Hemeproteins. J. Colloid Interface Sci. 2019, 541, 1–11. [Google Scholar] [CrossRef]
- Li, K.; Shi, Y.; Jia, F.; Price, C.; Gong, Y.; Huang, J.; Copner, N.; Cao, H.; Yang, L.; Chen, S. Low Etendue Yellow-Green Solid-State Light Generation by Laser-Pumped LuAG: Ce Ceramic. IEEE Photonics Technol. Lett. 2018, 30, 939–942. [Google Scholar] [CrossRef]
- Longcore, T.; Rodríguez, A.; Witherington, B.; Penniman, J.F.; Herf, L.; Herf, M. Rapid Assessment of Lamp Spectrum to Quantify Ecological Effects of Light at Night. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2018, 329, 511–521. [Google Scholar] [CrossRef]
- Kaiser, K.; Gross, L.; Schulz, F. A Single-Molecule Chemical Reaction Studied by High-Resolution Atomic Force Microscopy and Scanning Tunneling Microscopy Induced Light Emission. ACS Nano 2019, 13, 6947–6954. [Google Scholar] [CrossRef]
- Gao, J. Polymer Light-Emitting Electrochemical Cells—Recent Advances and Future Trends. Curr. Opin. Electrochem. 2018, 7, 87–94. [Google Scholar] [CrossRef]
- Forbes, A. Structured Light from Lasers. Laser Photonics Rev. 2019, 13, 1900140. [Google Scholar] [CrossRef]
- Zhang, F.; Santos, H.A. Photosensitive materials for constructing on-demanded drug-release systems. In Photoactive Inorganic Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 193–210. [Google Scholar]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef] [Green Version]
- Alster, T.S.; Li, M.K. Microneedling Treatment of Striae Distensae in Light and Dark Skin with Long-Term Follow-Up. Dermatol. Surg. 2020, 46, 459–464. [Google Scholar] [CrossRef]
- Tipirneni, K.E.; Warram, J.M.; Moore, L.S.; Prince, A.C.; de Boer, E.; Jani, A.H.; Wapnir, I.L.; Liao, J.C.; Bouvet, M.; Behnke, N.K. Oncologic Procedures Amenable to Fluorescence-Guided Surgery. Ann. Surg. 2017, 266, 36. [Google Scholar] [CrossRef] [Green Version]
- Palombo, F.; Fioretto, D. Brillouin Light Scattering: Applications in Biomedical Sciences. Chem. Rev. 2019, 119, 7833–7847. [Google Scholar] [CrossRef] [Green Version]
- Szeto, W.; Yam, W.C.; Huang, H.; Leung, D.Y.C. The Efficacy of Vacuum-Ultraviolet Light Disinfection of Some Common Environmental Pathogens. BMC Infect. Dis. 2020, 20, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Gao, Q.; Al-Enizi, A.M.; Nafady, A.; Ma, S. Recent Advances in MOF-Based Photocatalysis: Environmental Remediation under Visible Light. Inorg. Chem. Front. 2020, 7, 300–339. [Google Scholar] [CrossRef]
- Nguyen, D.M.; Lee, D.; Rho, J. Control of Light Absorbance Using Plasmonic Grating Based Perfect Absorber at Visible and Near-Infrared Wavelengths. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bang, W.; Jungfleisch, M.B.; Lim, J.; Trossman, J.; Tsai, C.C.; Hoffmann, A.; Ketterson, J.B. Excitation of the Three Principal Spin Waves in Yttrium Iron Garnet Using a Wavelength-Specific Multi-Element Antenna. AIP Adv. 2018, 8, 056015. [Google Scholar] [CrossRef] [Green Version]
- Kneissl, M.; Seong, T.-Y.; Han, J.; Amano, H. The Emergence and Prospects of Deep-Ultraviolet Light-Emitting Diode Technologies. Nat. Photonics 2019, 13, 233–244. [Google Scholar] [CrossRef]
- Singh, S.; Nannuri, S.H.; George, S.D.; Chakraborty, S.; Sharma, A.; Misra, S.K. Enhanced Visible/NIR Driven Catalytic Activity in Presence of Neodymium (Nd3+), for Yb3+ and Tm3+ Doped NaYF4 Nanoparticles. J. Environ. Chem. Eng. 2021, 9, 105813. [Google Scholar] [CrossRef]
- Costa, D.F.; Mendes, L.P.; Torchilin, V.P. The Effect of Low-and High-Penetration Light on Localized Cancer Therapy. Adv. Drug Deliv. Rev. 2019, 138, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Aw, J.; Xing, B. Nanostructures for NIR Light-Controlled Therapies. Nanoscale 2017, 9, 3698–3718. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, G. NIR Light-Responsive Nanocarriers for Controlled Release. J. Photochem. Photobiol. C Photochem. Rev. 2021, 47, 100420. [Google Scholar] [CrossRef]
- Shaabani, E.; Sharifiaghdam, M.; de Keersmaecker, H.; de Rycke, R.; de Smedt, S.; Faridi-Majidi, R.; Braeckmans, K.; Fraire, J.C. Layer by Layer Assembled Chitosan-Coated Gold Nanoparticles for Enhanced SiRNA Delivery and Silencing. Int. J. Mol. Sci. 2021, 22, 831. [Google Scholar] [CrossRef] [PubMed]
- Mahata, M.K.; De, R.; Lee, K.T. Near-Infrared-Triggered Upconverting Nanoparticles for Biomedicine Applications. Biomedicines 2021, 9, 756. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Kierulf, A.; Abbaspourrad, A. Light-Harvesting Synthetic Nano-and Micromotors: A Review. Nanoscale 2017, 9, 12218–12230. [Google Scholar] [CrossRef]
- Giménez, V.M.M.; Arya, G.; Succhi, I.A.; Galante, M.J.; Manucha, W. Photo-Responsive Polymeric Nanocarriers for Target-Specific and Controlled Drug Delivery. Soft Matter 2021, 17, 8577–8584. [Google Scholar] [CrossRef]
- Chang, D.; Ma, Y.; Xu, X.; Xie, J.; Ju, S. Stimuli-Responsive Polymeric Nanoplatforms for Cancer Therapy. Front. Bioeng. Biotechnol. 2021, 9, 528. [Google Scholar] [CrossRef]
- Cao, S.; Shao, J.; Wu, H.; Song, S.; de Martino, M.T.; Pijpers, I.A.B.; Friedrich, H.; Abdelmohsen, L.K.E.A.; Williams, D.S.; van Hest, J.C.M. Photoactivated Nanomotors via Aggregation Induced Emission for Enhanced Phototherapy. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Uygun, D.A.; Jurado-Sánchez, B.; Uygun, M.; Wang, J. Self-Propelled Chelation Platforms for Efficient Removal of Toxic Metals. Environ. Sci. Nano 2016, 3, 559–566. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, Protection, and Delivery of Bioactive Proteins and Peptides Using Nanoparticle and Microparticle Systems: A Review. Adv. Colloid Interface Sci. 2018, 253, 1–22. [Google Scholar] [CrossRef]
- Naik, J.B.; Pardeshi, S.R.; Patil, R.P.; Patil, P.B.; Mujumdar, A. Mucoadhesive Micro-/Nano Carriers in Ophthalmic Drug Delivery: An Overview. BioNanoScience 2020, 10, 564–582. [Google Scholar] [CrossRef]
- Zhang, P.; Yue, L.; Vázquez-Gonzaález, M.; Zhou, Z.; Chen, W.-H.; Sohn, Y.S.; Nechushtai, R.; Willner, I. MicroRNA-Guided Selective Release of Loads from Micro-/Nanocarriers Using Auxiliary Constitutional Dynamic Networks. ACS Nano 2020, 14, 1482–1491. [Google Scholar] [CrossRef]
- Comunian, T.; Babazadeh, A.; Rehman, A.; Shaddel, R.; Akbari-Alavijeh, S.; Boostani, S.; Jafari, S.M. Protection and Controlled Release of Vitamin C by Different Micro/Nanocarriers; Taylor & Francis: England, UK, 2020; pp. 1–22. [Google Scholar]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Majumder, N.; G Das, N.; Das, S.K. Polymeric Micelles for Anticancer Drug Delivery. Ther. Deliv. 2020, 11, 613–635. [Google Scholar] [CrossRef]
- Lefley, J.; Waldron, C.; Becer, C.R. Macromolecular Design and Preparation of Polymersomes. Polym. Chem. 2020, 11, 7124–7136. [Google Scholar] [CrossRef]
- Ramezanli, T.; Kilfoyle, B.E.; Zhang, Z.; Michniak-Kohn, B.B. Polymeric Nanospheres for Topical Delivery of Vitamin D3. Int. J. Pharm. 2017, 516, 196–203. [Google Scholar] [CrossRef] [Green Version]
- Rivas, C.J.M.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Rodríguez, S.A.G.; Román, R.Á.; Fessi, H.; Elaissari, A. Nanoprecipitation Process: From Encapsulation to Drug Delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef]
- Kumar, N.; Mandal, A. Oil-in-Water Nanoemulsion Stabilized by Polymeric Surfactant: Characterization and Properties Evaluation for Enhanced Oil Recovery. Eur. Polym. J. 2018, 109, 265–276. [Google Scholar] [CrossRef]
- Mavila, S.; Eivgi, O.; Berkovich, I.; Lemcoff, N.G. Intramolecular Cross-Linking Methodologies for the Synthesis of Polymer Nanoparticles. Chem. Rev. 2016, 116, 878–961. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, B.; Ren, Y.; Wang, Z.; Zhao, C.-X.; Hai, M.; Weitz, D.A.; Chen, D. Diverse Particle Carriers Prepared by Co-Precipitation and Phase Separation: Formation and Applications. ChemPlusChem 2020, 85, 1–11. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Lange, H.; Crestini, C.; Henn, A.; Österberg, M. Lignin for Nano-and Microscaled Carrier Systems: Applications, Trends, and Challenges. ChemSusChem 2019, 12, 2039. [Google Scholar] [CrossRef]
- Sánchez, A.; Mejía, S.P.; Orozco, J. Recent Advances in Polymeric Nanoparticle-Encapsulated Drugs against Intracellular Infections. Molecules 2020, 25, 3760. [Google Scholar] [CrossRef]
- Yazdi, M.K.; Taghizadeh, A.; Taghizadeh, M.; Stadler, F.J.; Farokhi, M.; Mottaghitalab, F.; Zarrintaj, P.; Ramsey, J.D.; Seidi, F.; Saeb, M.R. Agarose-Based Biomaterials for Advanced Drug Delivery. J. Control. Release 2020, 326, 523–543. [Google Scholar] [CrossRef]
- Bui, T.; Phan, A.; Cole, D.R.; Striolo, A. Transport Mechanism of Guest Methane in Water-Filled Nanopores. J. Phys. Chem. C 2017, 121, 15675–15686. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.; Yu, Q.; Xie, Y.; Li, F.; Lu, Y.; Dong, X.; Zhao, W.; Qi, J.; Wu, W. Controlling Release of Integral Lipid Nanoparticles Based on Osmotic Pump Technology. Pharm. Res. 2016, 33, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Son, G.-H.; Lee, B.-J.; Cho, C.-W. Mechanisms of Drug Release from Advanced Drug Formulations Such as Polymeric-Based Drug-Delivery Systems and Lipid Nanoparticles. J. Pharm. Investig. 2017, 47, 287–296. [Google Scholar] [CrossRef]
- Liu, J.; Tan, C.S.Y.; Lan, Y.; Scherman, O.A. Aqueous Polymer Self-Assembly Based on Cucurbit [n] Uril-Mediated Host-Guest Interactions. Macromol. Chem. Phys. 2016, 217, 319–332. [Google Scholar] [CrossRef]
- Vauthier, C.; Ponchel, G. Polymer Nanoparticles for Nanomedicines; Springer: Berlin, Germany, 2017; ISBN 3319414194. [Google Scholar]
- Saucier-Sawyer, J.K.; Seo, Y.-E.; Gaudin, A.; Quijano, E.; Song, E.; Sawyer, A.J.; Deng, Y.; Huttner, A.; Saltzman, W.M. Distribution of Polymer Nanoparticles by Convection-Enhanced Delivery to Brain Tumors. J. Control. Release 2016, 232, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [Green Version]
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef] [Green Version]
- Chapman, R.; Stenzel, M.H. All Wrapped up: Stabilization of Enzymes within Single Enzyme Nanoparticles. J. Am. Chem. Soc. 2019, 141, 2754–2769. [Google Scholar] [CrossRef]
- Vázquez-Núñez, E.; Molina-Guerrero, C.E.; Peña-Castro, J.M.; Fernández-Luqueño, F.; de la Rosa-Álvarez, M. Use of Nanotechnology for the Bioremediation of Contaminants: A Review. Processes 2020, 8, 826. [Google Scholar] [CrossRef]
- Lee, K.M.; Kim, K.H.; Yoon, H.; Kim, H. Chemical Design of Functional Polymer Structures for Biosensors: From Nanoscale to Macroscale. Polymers 2018, 10, 551. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, N.L.; Kempe, K.; Thurecht, K.J. Next-generation Polymeric Nanomedicines for Oncology: Perspectives and Future Directions. Macromol. Rapid Commun. 2020, 41, 2000319. [Google Scholar] [CrossRef]
- Zhang, W.; Hong, C.; Pan, C. Polymerization-induced Self-assembly of Functionalized Block Copolymer Nanoparticles and Their Application in Drug Delivery. Macromol. Rapid Commun. 2019, 40, 1800279. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Z.; Fang, S.; Tang, Z.; Liu, F.; Guo, B. Malleable Organic/Inorganic Thermosetting Hybrids Enabled by Exchangeable Silyl Ether Interfaces. J. Mater. Chem. A 2019, 7, 1459–1467. [Google Scholar] [CrossRef]
- Pan, D.; Vargas-Morales, O.; Zern, B.; Anselmo, A.C.; Gupta, V.; Zakrewsky, M.; Mitragotri, S.; Muzykantov, V. The Effect of Polymeric Nanoparticles on Biocompatibility of Carrier Red Blood Cells. PLoS ONE 2016, 11, e0152074. [Google Scholar] [CrossRef] [Green Version]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Vahed, S.Z.; Salehi, R.; Davaran, S.; Sharifi, S. Liposome-Based Drug Co-Delivery Systems in Cancer Cells. Mater. Sci. Eng. C 2017, 71, 1327–1341. [Google Scholar] [CrossRef]
- Man, F.; Gawne, P.J.; de Rosales, R.T.M. Nuclear Imaging of Liposomal Drug Delivery Systems: A Critical Review of Radiolabelling Methods and Applications in Nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 134–160. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Gudbergsson, J.M.; Duroux, M.; Moos, T.; Andresen, T.L.; Simonsen, J.B. On the Use of Liposome Controls in Studies Investigating the Clinical Potential of Extracellular Vesicle-Based Drug Delivery Systems—A Commentary. J. Control. Release 2018, 269, 10–14. [Google Scholar] [CrossRef] [PubMed]
- El-Hammadi, M.M.; Arias, J.L. An Update on Liposomes in Drug Delivery: A Patent Review (2014–2018). Expert Opin. Ther. Pat. 2019, 29, 891–907. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zheng, H.; Guo, Y.; Wang, Y.; Anderson, G.J.; Ci, Y.; Yu, P.; Geng, L.; Chang, Y.-Z. Nasal Delivery of Nanoliposome-Encapsulated Ferric Ammonium Citrate Can Increase the Iron Content of Rat Brain. J. Nanobiotechnology 2017, 15, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mejía De Los Ríos, S.P.; Sánchez Toro, A.; Vásquez, V.; Orozco Holguín, J. Functional Nanocarriers for Delivering Itraconazole against Fungal Intracellular Infections. Front. Pharmacol. 2021, 12, 1520. [Google Scholar]
- Khan, R.U.; Yu, H.; Wang, L.; Zhang, Q.; Xiong, W.; Nazir, A.; Fahad, S.; Chen, X.; Elsharaarani, T. Synthesis of Polyorganophosphazenes and Preparation of Their Polymersomes for Reductive/Acidic Dual-Responsive Anticancer Drugs Release. J. Mater. Sci. 2020, 55, 8264–8284. [Google Scholar] [CrossRef]
- Cerón, A.A.; Nascife, L.; Norte, S.; Costa, S.A.; do Nascimento, J.H.O.; Morisso, F.D.P.; Baruque-Ramos, J.; Oliveira, R.C.; Costa, S.M. Synthesis of Chitosan-Lysozyme Microspheres, Physicochemical Characterization, Enzymatic and Antimicrobial Activity. Int. J. Biol. Macromol. 2021, 185, 572–581. [Google Scholar] [CrossRef]
- Moffitt, M.; Khougaz, K.; Eisenberg, A. Micellization of Ionic Block Copolymers. Acc. Chem. Res. 1996, 29, 95–102. [Google Scholar] [CrossRef]
- Manjappa, A.S.; Kumbhar, P.S.; Patil, A.B.; Disouza, J.I.; Patravale, V.B. Polymeric Mixed Micelles: Improving the Anticancer Efficacy of Single-Copolymer Micelles. Crit. Rev. Ther. Drug Carr. Syst. 2019, 36, 1–58. [Google Scholar] [CrossRef]
- Lu, Y.; Yue, Z.; Xie, J.; Wang, W.; Zhu, H.; Zhang, E.; Cao, Z. Micelles with Ultralow Critical Micelle Concentration as Carriers for Drug Delivery. Nat. Biomed. Eng. 2018, 2, 318–325. [Google Scholar] [CrossRef]
- Umapathi, R.; Venkatesu, P. Thermo-Responsive Triblock Copolymer Phase Transition Behaviour in Imidazolium-Based Ionic Liquids: Role of the Effect of Alkyl Chain Length of Cations. J. Colloid Interface Sci. 2017, 485, 183–191. [Google Scholar] [CrossRef]
- Kauscher, U.; Holme, M.N.; Björnmalm, M.; Stevens, M.M. Physical Stimuli-Responsive Vesicles in Drug Delivery: Beyond Liposomes and Polymersomes. Adv. Drug Deliv. Rev. 2019, 138, 259–275. [Google Scholar] [CrossRef]
- Oz, U.C.; Kucukturkmen, B.; Ozkose, U.U.; Gulyuz, S.; Bolat, Z.B.; Telci, D.; Sahin, F.; Yilmaz, O.; Bozkir, A. Design of Colloidally Stable and Non-Toxic Petox-Based Polymersomes for Cargo Molecule Encapsulation. ChemNanoMat 2019, 5, 766–775. [Google Scholar] [CrossRef]
- Iatridi, Z.; Angelopoulou, A.; Voulgari, E.; Avgoustakis, K.; Tsitsilianis, C. Star-Graft Quarterpolymer-Based Polymersomes as Nanocarriers for Co-Delivery of Hydrophilic/Hydrophobic Chemotherapeutic Agents. ACS Omega 2018, 3, 11896–11908. [Google Scholar] [CrossRef]
- Leong, J.; Teo, J.Y.; Aakalu, V.K.; Yang, Y.Y.; Kong, H. Engineering Polymersomes for Diagnostics and Therapy. Adv. Healthc. Mater. 2018, 7, 1701276. [Google Scholar] [CrossRef]
- Lei, L.; Bergstrom, D.; Zhang, B.; Zhang, H.; Yin, R.; Song, K.-Y.; Zhang, W. Micro/Nanospheres Generation by Fluid-Fluid Interaction Technology: A Literature Review. Recent Pat. Nanotechnol. 2017, 11, 15–33. [Google Scholar] [CrossRef]
- Abdelkarim, M.; Abd Ellah, N.H.; Elsabahy, M.; Abdelgawad, M.; Abouelmagd, S.A. Microchannel Geometry vs Flow Parameters for Controlling Nanoprecipitation of Polymeric Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125774. [Google Scholar] [CrossRef]
- Zhai, L.; Bai, Z.; Zhu, Y.; Wang, B.; Luo, W. Fabrication of Chitosan Microspheres for Efficient Adsorption of Methyl Orange. Chin. J. Chem. Eng. 2018, 26, 657–666. [Google Scholar] [CrossRef]
- Tian, Q.; Fei, C.; Yin, H.; Feng, Y. Stimuli-Responsive Polymer Wormlike Micelles. Prog. Polym. Sci. 2019, 89, 108–132. [Google Scholar] [CrossRef]
- Iyer, R.; Wolf, J.; Zhukova, D.; Padanilam, D.; Nguyen, K.T. Nanomaterial Based Photo-Triggered Drug Delivery Strategies for Cancer Theranostics. In Handbook of Nanomaterials for Cancer Theranostics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 351–391. [Google Scholar]
- Zhang, X.; Ma, X.; Wang, K.; Lin, S.; Zhu, S.; Dai, Y.; Xia, F. Recent Advances in Cyclodextrin-based Light-responsive Supramolecular Systems. Macromol. Rapid Commun. 2018, 39, 1800142. [Google Scholar] [CrossRef]
- Linsley, C.S.; Wu, B.M. Recent Advances in Light-Responsive on-Demand Drug-Delivery Systems. Ther. Deliv. 2017, 8, 89–107. [Google Scholar] [CrossRef] [Green Version]
- Verde-Sesto, E.; Blázquez-Martín, A.; Pomposo, J.A. Advances in the Phototriggered Synthesis of Single-Chain Polymer Nanoparticles. Polymers 2019, 11, 1903. [Google Scholar] [CrossRef] [Green Version]
- Marturano, V.; Cerruti, P.; Giamberini, M.; Tylkowski, B.; Ambrogi, V. Light-Responsive Polymer Micro-and Nano-Capsules. Polymers 2017, 9, 8. [Google Scholar] [CrossRef]
- Yang, L.; Sun, H.; Liu, Y.; Hou, W.; Yang, Y.; Cai, R.; Cui, C.; Zhang, P.; Pan, X.; Li, X. Self-assembled Aptamer-grafted Hyperbranched Polymer Nanocarrier for Targeted and Photoresponsive Drug Delivery. Angew. Chem. 2018, 130, 17294–17298. [Google Scholar] [CrossRef]
- Appavoo, D.; Park, S.Y.; Zhai, L. Responsive Polymers for Medical Diagnostics. J. Mater. Chem. B 2020, 8, 6217–6232. [Google Scholar] [CrossRef]
- Tamay, D.G.; Dursun Usal, T.; Alagoz, A.S.; Yucel, D.; Hasirci, N.; Hasirci, V. 3D and 4D Printing of Polymers for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2019, 7, 164. [Google Scholar] [CrossRef]
- Xie, Y.; Tuguntaev, R.G.; Mao, C.; Chen, H.; Tao, Y.; Wang, S.; Yang, B.; Guo, W. Stimuli-Responsive Polymeric Nanomaterials for Rheumatoid Arthritis Therapy. Biophys. Rep. 2020, 6, 1–18. [Google Scholar] [CrossRef]
- Huang, B.; Chen, F.; Shen, Y.; Qian, K.; Wang, Y.; Sun, C.; Zhao, X.; Cui, B.; Gao, F.; Zeng, Z. Advances in Targeted Pesticides with Environmentally Responsive Controlled Release by Nanotechnology. Nanomaterials 2018, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Yang, Y.; Urban, M.W. Stimuli-responsive Polymeric Nanoparticles. Macromol. Rapid Commun. 2017, 38, 1700030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghani, M.; Heiskanen, A.; Kajtez, J.; Rezaei, B.; Larsen, N.B.; Thomsen, P.; Kristensen, A.; Žukauskas, A.; Alm, M.; Emnéus, J. On-Demand Reversible UV-Triggered Interpenetrating Polymer Network-Based Drug Delivery System Using the Spiropyran–Merocyanine Hydrophobicity Switch. ACS Appl. Mater. Interfaces 2021, 13, 3591–3604. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ye, H.; Chen, Y.; Zhu, R.; Yin, L. Photoresponsive Drug/Gene Delivery Systems. Biomacromolecules 2018, 19, 1840–1857. [Google Scholar] [CrossRef] [PubMed]
- Weis, P.; Wu, S. Light-switchable Azobenzene-containing Macromolecules: From UV to near Infrared. Macromol. Rapid Commun. 2018, 39, 1700220. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhang, Q.; Zhou, Y.-N.; Zhu, S. Let Spiropyran Help Polymers Feel Force! Prog. Polym. Sci. 2018, 79, 26–39. [Google Scholar] [CrossRef]
- Guo, X.; You, J. Near Infrared Light-Controlled Therapeutic Molecules Release of Nanocarriers in Cancer Therapy. J. Pharm. Investig. 2017, 47, 297–316. [Google Scholar] [CrossRef]
- Wong, P.T.; Tang, S.; Cannon, J.; Chen, D.; Sun, R.; Lee, J.; Phan, J.; Tao, K.; Sun, K.; Chen, B.; et al. Photocontrolled Release of Doxorubicin Conjugated through a Thioacetal Photocage in Folate-Targeted Nanodelivery Systems. Bioconjugate Chem. 2017, 28, 3016–3028. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Zheng, J.; Zhou, Z.; Zhang, D. Approaches for the Synthesis of O-Nitrobenzyl and Coumarin Linkers for Use in Photocleavable Biomaterials and Bioconjugates and Their Biomedical Applications. Acta Biomater. 2020, 115, 75–91. [Google Scholar] [CrossRef]
- Negri, G.E.; Deming, T.J. Triggered Copolypeptide Hydrogel Degradation Using Photolabile Lysine Protecting Groups. ACS Macro Lett. 2016, 5, 1253–1256. [Google Scholar] [CrossRef] [Green Version]
- Katz, J.S.; Burdick, J.A. Light-responsive Biomaterials: Development and Applications. Macromol. Biosci. 2010, 10, 339–348. [Google Scholar] [CrossRef]
- Jiang, M.; Paul, N.; Bieniek, N.; Buckup, T.; Hampp, N.; Motzkus, M. Photocleavage of Coumarin Dimers Studied by Femtosecond UV Transient Absorption Spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 4597–4606. [Google Scholar] [CrossRef]
- Feng, S.; Wang, J.; Zhang, L.; Chen, Q.; Yue, W.; Ke, N.; Xie, H. Coumarin-Containing Light-Responsive Carboxymethyl Chitosan Micelles as Nanocarriers for Controlled Release of Pesticide. Polymers 2020, 12, 2268. [Google Scholar] [CrossRef]
- Lale, S.V.; Koul, V. Stimuli-responsive polymeric nanoparticles for cancer therapy. In Polymer Gels; Springer: Berlin, Germany, 2018; pp. 27–54. [Google Scholar]
- Xiong, Q.; Lim, Y.; Li, D.; Pu, K.; Liang, L.; Duan, H. Photoactive Nanocarriers for Controlled Delivery. Adv. Funct. Mater. 2020, 30, 1903896. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, Z.; Shen, J.; Hu, J.; Niu, Y.; Xiao, Z.; Chen, L.; Zhang, X. Preparation of Photo-Responsive Nanomicelles Loaded with Sandela 803 for Wallpaper Application. Nanosci. Nanotechnol. Lett. 2019, 11, 22–30. [Google Scholar] [CrossRef]
- Hou, W.; Liu, R.; Bi, S.; He, Q.; Wang, H.; Gu, J. Photo-Responsive Polymersomes as Drug Delivery System for Potential Medical Applications. Molecules 2020, 25, 5147. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Huang, W.; Wang, D.; Huang, X.; Zhu, X.; Yan, D. Chitosan-Based Nanocarriers with PH and Light Dual Response for Anticancer Drug Delivery. Biomacromolecules 2013, 14, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Buitrago, S.; Mena-Giraldo, P.; Pinal, R.; Hoyos-Palacio, L. Azopolymer Based Nanoparticles for Phototriggered Drug Delivery. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 1089–1092. [Google Scholar]
- Soliman, S.M.A.; el Founi, M.; Vanderesse, R.; Acherar, S.; Ferji, K.; Babin, J.; Six, J.-L. Light-Sensitive Dextran-Covered PNBA Nanoparticles to Continuously or Discontinuously Improve the Drug Release. Colloids Surf. B Biointerfaces 2019, 182, 110393. [Google Scholar] [CrossRef]
- Tunc, D.; le Coz, C.; Alexandre, M.; Desbois, P.; Lecomte, P.; Carlotti, S. Reversible Cross-Linking of Aliphatic Polyamides Bearing Thermo-and Photoresponsive Cinnamoyl Moieties. Macromolecules 2014, 47, 8247–8254. [Google Scholar] [CrossRef]
- Akiba, U.; Minaki, D.; Anzai, J. Photosensitive Layer-by-Layer Assemblies Containing Azobenzene Groups: Synthesis and Biomedical Applications. Polymers 2017, 9, 553. [Google Scholar] [CrossRef] [Green Version]
- Mena-Giraldo, P.; Pérez-Buitrago, S.; Londoño-Berrío, M.; Ortiz-Trujillo, I.C.; Hoyos-Palacio, L.M.; Orozco, J. Photosensitive Nanocarriers for Specific Delivery of Cargo into Cells. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Molla, M.R.; Rangadurai, P.; Antony, L.; Swaminathan, S.; de Pablo, J.J.; Thayumanavan, S. Dynamic Actuation of Glassy Polymersomes through Isomerization of a Single Azobenzene Unit at the Block Copolymer Interface. Nat. Chem. 2018, 10, 659–666. [Google Scholar] [CrossRef]
- Razavi, B.; Abdollahi, A.; Roghani-Mamaqani, H.; Salami-Kalajahi, M. Light-and Temperature-Responsive Micellar Carriers Prepared by Spiropyran-Initiated Atom Transfer Polymerization: Investigation of Photochromism Kinetics, Responsivities, and Controlled Release of Doxorubicin. Polymer 2020, 187, 122046. [Google Scholar] [CrossRef]
- Cao, J.; Huang, S.; Chen, Y.; Li, S.; Li, X.; Deng, D.; Qian, Z.; Tang, L.; Gu, Y. Near-Infrared Light-Triggered Micelles for Fast Controlled Drug Release in Deep Tissue. Biomaterials 2013, 34, 6272–6283. [Google Scholar] [CrossRef]
- Doi, N.; Yamauchi, Y.; Ikegami, R.; Kuzuya, M.; Sasai, Y.; Kondo, S. Photo-Responsive Polymer Micelles from o-Nitrobenzyl Ester-Based Amphiphilic Block Copolymers Synthesized by Mechanochemical Solid-State Copolymerization. Polym. J. 2020, 52, 1375–1385. [Google Scholar] [CrossRef]
- Jiang, J.; Tong, X.; Zhao, Y. A New Design for Light-Breakable Polymer Micelles. J. Am. Chem. Soc. 2005, 127, 8290–8291. [Google Scholar] [CrossRef]
- Arjmand, F.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Preparation of Photolabile Nanoparticles by Coumarin-Based Crosslinker for Drug Delivery under Light Irradiation. J. Phys. Chem. Solids 2021, 154, 110102. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, J.; Zhao, J.; Stenzel, M.H. Light-Induced Release of Molecules from Polymers. Prog. Polym. Sci. 2017, 74, 1–33. [Google Scholar] [CrossRef]
- Roche, A.; Oriol, L.; Tejedor, R.M.; Piñol, M. Polymeric Self-Assemblies Based on Tetra-Ortho-Substituted Azobenzene as Visible Light Responsive Nanocarriers. Polymers 2019, 11, 2060. [Google Scholar] [CrossRef] [Green Version]
- Huo, H.; Ma, X.; Dong, Y.; Qu, F. Light/Temperature Dual-Responsive ABC Miktoarm Star Terpolymer Micelles for Controlled Release. Eur. Polym. J. 2017, 87, 331–343. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, X.; Liang, T.; Tong, Z. Acid/Light Dual-Responsive Biodegradable Polymeric Nanocarriers for Efficient Intracellular Drug Delivery. Polym. Bull. 2019, 76, 1775–1792. [Google Scholar] [CrossRef]
- Razavi, B.; Abbaszadeh, R.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Multi-Responsive Poly (Amidoamine)-Initiated Dendritic-Star Supramolecular Structures Containing UV Cross-Linkable Coumarin Groups for Smart Drug Delivery. J. Mol. Liq. 2020, 319, 114138. [Google Scholar] [CrossRef]
- Mukherjee, A.; Ghosh, S. Phototriggered Supramolecular Assembly. ACS Omega 2020, 5, 32140–32148. [Google Scholar] [CrossRef]
- Dutta, A. Fourier Transform Infrared Spectroscopy. Spectrosc. Methods Nanomater. Charact. 2017, 73–93. [Google Scholar]
- Novais, Â.; Freitas, A.R.; Rodrigues, C.; Peixe, L. Fourier Transform Infrared Spectroscopy: Unlocking Fundamentals and Prospects for Bacterial Strain Typing. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 427–448. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, X.; Nie, B.; Song, D. FTIR and Raman Spectroscopy Characterization of Functional Groups in Various Rank Coals. Fuel 2017, 206, 555–563. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Hong, C.-Y.; Pan, C.-Y. Efficient Fabrication of Photosensitive Polymeric Nano-Objects via an Ingenious Formulation of RAFT Dispersion Polymerization and Their Application for Drug Delivery. Biomacromolecules 2017, 18, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Huang, Y.; Lu, H.; Qiu, T.; Guo, D.; Agback, T.; Orekhov, V.; Chen, Z. Accelerated Nuclear Magnetic Resonance Spectroscopy with Deep Learning. Angew. Chem. 2020, 132, 10383–10386. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.; Burgeson, I.E.; Adami, S.R.; Sinkov, S.I. Isotope-specific Analysis of Neutron-irradiated Lithium Aluminate Ceramics by Nuclear Magnetic Resonance Spectroscopy. J. Am. Ceram. Soc. 2020, 103, 7291–7298. [Google Scholar] [CrossRef]
- Lambert, J.B.; Mazzola, E.P.; Ridge, C.D. Nuclear Magnetic Resonance Spectroscopy: An Introduction to Principles, Applications, and Experimental Methods; John Wiley & Sons: Hoboken, NJ, USA, 2019; ISBN 1119295238. [Google Scholar]
- Mahy, R.; Oulmidi, A.; CHALLIOUI, A. Photocrosslinked Polymer Based on Pendant Extended Cinnamate as Photoreactive Moieties. Chem. Res. J. 2018, 3, 130–139. [Google Scholar]
- Laan, A.C.; Denkova, A.G. Cryogenic Transmission Electron Microscopy: The Technique of Choice for the Characterization of Polymeric Nanocarriers. EJNMMI Res. 2017, 7, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haggag, Y.A.; Yasser, M.; Tambuwala, M.M.; el Tokhy, S.S.; Isreb, M.; Donia, A.A. Repurposing of Guanabenz Acetate by Encapsulation into Long-Circulating Nanopolymersomes for Treatment of Triple-Negative Breast Cancer. Int. J. Pharm. 2021, 600, 120532. [Google Scholar] [CrossRef]
- Weng, Y.-H.; Che, J.; Ma, X.-W.; Guo, H.-B.; Xue, X.-D.; Chen, S.-Z.; Zhang, X.; Zhang, T.-B.; Li, C.; Xu, J. Contrast Enhancement Method of Transmission Electron Microscopy in Visualization of Polymeric Micelles by Fluoride Addition and Staining. J. Biomed. Nanotechnol. 2017, 13, 534–543. [Google Scholar] [CrossRef]
- Allen, S.D.; Bobbala, S.; Karabin, N.B.; Modak, M.; Scott, E.A. Benchmarking Bicontinuous Nanospheres against Polymersomes for in Vivo Biodistribution and Dual Intracellular Delivery of Lipophilic and Water-Soluble Payloads. ACS Appl. Mater. Interfaces 2018, 10, 33857–33866. [Google Scholar] [CrossRef]
- Akhtar, K.; Khan, S.A.; Khan, S.B.; Asiri, A.M. Scanning electron microscopy: Principle and applications in nanomaterials characterization. In Handbook of Materials Characterization; Springer: Berlin, Germany, 2018; pp. 113–145. [Google Scholar]
- Falsafi, S.R.; Rostamabadi, H.; Assadpour, E.; Jafari, S.M. Morphology and Microstructural Analysis of Bioactive-Loaded Micro/Nanocarriers via Microscopy Techniques; CLSM/SEM/TEM/AFM. Adv. Colloid Interface Sci. 2020, 102166. [Google Scholar] [CrossRef]
- Falke, S.; Betzel, C. Dynamic Light Scattering (DLS). In Radiation in Bioanalysis; Springer: Berlin, Germany, 2019; pp. 173–193. [Google Scholar]
- Dinari, A.; Farsani, S.S.M.; Mohammadi, S.; Najafi, F.; Abdollahi, M. Facile Method for Morphological Characterization at Nano Scale. Iran. J. Biotechnol. 2020, 18, e2645. [Google Scholar]
- Chroni, A.; Mavromoustakos, T.; Pispas, S. Nano-Assemblies from Amphiphilic PnBA-b-POEGA Copolymers as Drug Nanocarriers. Polymers 2021, 13, 1164. [Google Scholar] [CrossRef]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta Potential: A Case Study of Cationic, Anionic, and Neutral Liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef]
- Manaia, E.B.; Abuçafy, M.P.; Chiari-Andréo, B.G.; Silva, B.L.; Junior, J.A.O.; Chiavacci, L.A. Physicochemical Characterization of Drug Nanocarriers. Int. J. Nanomed. 2017, 12, 4991. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-F.; Li, C.-Y.; Aroca, R.F. Plasmon-Enhanced Fluorescence Spectroscopy. Chem. Soc. Rev. 2017, 46, 3962–3979. [Google Scholar] [CrossRef]
- Bell, J.E.; Hall, C. UV and visible absorbance spectroscopy. In Spectroscopy in Biochemistry; CRC Press: Boca Raton, FL, USA, 2018; pp. 3–62. ISBN 1351076825. [Google Scholar]
- Xu, Y.; Cao, J.; Li, Q.; Li, J.; He, K.; Shen, T.; Liu, X.; Yuan, C.; Zeng, B.; Dai, L. Novel Azobenzene-Based Amphiphilic Copolymers: Synthesis, Self-Assembly Behavior and Multiple-Stimuli-Responsive Properties. RSC Adv. 2018, 8, 16103–16113. [Google Scholar] [CrossRef] [Green Version]
- Harvey, D. Modern Analytical Chemistry; McGraw-Hill: New York, NY, USA, 2000; Volume 1. [Google Scholar]
- Widmer, M. Gravimetry. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; John Wiley & Sons, Ltd.: Basel, Switzerland, 2006; pp. 1–6. [Google Scholar]
- Takeuchi, M. Titrimetry. Anal. Sci. 2021, 37, 227. [Google Scholar] [CrossRef]
- Kolthoff, I.M.; Menzel, H. Volumetric Analysis; CUP Archive: Cambridge, UK, 1929; Volume 1. [Google Scholar]
- Nagasawa, M.; Murase, T.; Kondo, K. Potentiometric Titration of Stereoregular Polyelectrolytes. J. Phys. Chem. 1965, 69, 4005–4012. [Google Scholar] [CrossRef]
- Sujata, V.; Ravishankar, G.A.; Venkataraman, L. v Methods for the Analysis of the Saffron Metabolites Crocin, Crocetins, Picrocrocin and Safranal for the Determination of the Quality of the Spice Using Thin-Layer Chromatography, High-Performance Liquid Chromatography and Gas Chromatography. J. Chromatogr. A 1992, 624, 497–502. [Google Scholar] [CrossRef]
- Basha, M. Chromatography. In Analytical Techniques in Biochemistry; Springer: Berlin, Germany, 2020; pp. 39–59. [Google Scholar]
- Allen, S.D.; Liu, Y.-G.; Kim, T.; Bobbala, S.; Yi, S.; Zhang, X.; Choi, J.; Scott, E.A. Celastrol-Loaded PEG-b-PPS Nanocarriers as an Anti-Inflammatory Treatment for Atherosclerosis. Biomater. Sci. 2019, 7, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Oh, K.S.; Shim, J.; Schlaepfer, I.R.; Karam, S.D.; Lee, J.-J. Light-Responsive Polymeric Micellar Nanoparticles with Enhanced Formulation Stability. Polymers 2021, 13, 377. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, M.; Bennici, S.; Brendle, J.; Dutournie, P.; Limousy, L.; Pluchon, S. Systems for Stimuli-Controlled Release: Materials and Applications. J. Control. Release 2019, 294, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Pirone, D.; Marturano, V.; del Pezzo, R.; Fernández Prieto, S.; Underiner, T.; Giamberini, M.; Tylkowski, B. Molecular Design of Microcapsule Shells for Visible Light-Triggered Release. Polymers 2019, 11, 904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carnell-Morris, P.; Tannetta, D.; Siupa, A.; Hole, P.; Dragovic, R. Analysis of extracellular vesicles using fluorescence nanoparticle tracking analysis. In Extracellular Vesicles; Springer: Berlin, Germany, 2017; pp. 153–173. [Google Scholar]
- Aziz, A.; Medina-Sánchez, M.; Koukourakis, N.; Wang, J.; Kuschmierz, R.; Radner, H.; Czarske, J.W.; Schmidt, O.G. Real-time IR Tracking of Single Reflective Micromotors through Scattering Tissues. Adv. Funct. Mater. 2019, 29, 1905272. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ahmed, R.; Zeng, Y.; Fu, K.; Soto, F.; Sinclair, B.; Soh, H.T.; Demirci, U. Engineering the Interaction Dynamics between Nano-Topographical Immunocyte-Templated Micromotors across Scales from Ions to Cells. Small 2020, 16, 2005185. [Google Scholar] [CrossRef] [PubMed]
- Novotný, F.; Plutnar, J.; Pumera, M. Plasmonic Self-Propelled Nanomotors for Explosives Detection via Solution-Based Surface Enhanced Raman Scattering. Adv. Funct. Mater. 2019, 29, 1903041. [Google Scholar] [CrossRef]
- Bachurski, D.; Schuldner, M.; Nguyen, P.-H.; Malz, A.; Reiners, K.S.; Grenzi, P.C.; Babatz, F.; Schauss, A.C.; Hansen, H.P.; Hallek, M. Extracellular Vesicle Measurements with Nanoparticle Tracking Analysis–An Accuracy and Repeatability Comparison between NanoSight NS300 and ZetaView. J. Extracell. Vesicles 2019, 8, 1596016. [Google Scholar] [CrossRef] [PubMed]
- Karthik, S.; Jana, A.; Selvakumar, M.; Venkatesh, Y.; Paul, A.; Shah, S.S.; Singh, N.D.P. Coumarin Polycaprolactone Polymeric Nanoparticles: Light and Tumor Microenvironment Activated Cocktail Drug Delivery. J. Mater. Chem. B 2017, 5, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Ramazani, F.; Chen, W.; van Nostrum, C.F.; Storm, G.; Kiessling, F.; Lammers, T.; Hennink, W.E.; Kok, R.J. Strategies for Encapsulation of Small Hydrophilic and Amphiphilic Drugs in PLGA Microspheres: State-of-the-Art and Challenges. Int. J. Pharm. 2016, 499, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C.; McClements, D.J. Improving Curcumin Solubility and Bioavailability by Encapsulation in Saponin-Coated Curcumin Nanoparticles Prepared Using a Simple PH-Driven Loading Method. Food Funct. 2018, 9, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, A.; Liu, Q.; Kohane, D.S. Ultraviolet Light-Mediated Drug Delivery: Principles, Applications, and Challenges. J. Control. Release 2015, 219, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jung, J.; Zhao, Y. Chitosan-Cellulose Nanocrystal Microencapsulation to Improve Encapsulation Efficiency and Stability of Entrapped Fruit Anthocyanins. Carbohydr. Polym. 2017, 157, 1246–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Zhang, J.; Chen, Q.; Luo, Y.; Xu, F.; Chen, Y. Temperature and Photo Dual-Stimuli Responsive Block Copolymer Self-Assembly Micelles for Cellular Controlled Drug Release. Macromol. Biosci. 2021, 21, 2000291. [Google Scholar] [CrossRef]
- Kowalczuk, A.; Trzcinska, R.; Trzebicka, B.; Müller, A.H.E.; Dworak, A.; Tsvetanov, C.B. Loading of Polymer Nanocarriers: Factors, Mechanisms and Applications. Prog. Polym. Sci. 2014, 39, 43–86. [Google Scholar] [CrossRef]
- Abebe Alemayehu, Y.; Tewabe Gebeyehu, B.; Cheng, C.-C. Photosensitive Supramolecular Micelles with Complementary Hydrogen Bonding Motifs to Improve the Efficacy of Cancer Chemotherapy. Biomacromolecules 2019, 20, 4535–4545. [Google Scholar] [CrossRef]
- Ou, R.; Zhang, H.; Zhao, C.; Hegab, H.M.; Jiang, L.; Truong, V.X.; Wang, H. Photoresponsive Styrylpyrene-Modified MOFs for Gated Loading and Release of Cargo Molecules. Chem. Mater. 2020, 32, 10621–10627. [Google Scholar] [CrossRef]
- Xiong, H.; Li, X.; Kang, P.; Perish, J.; Neuhaus, F.; Ploski, J.E.; Kroener, S.; Ogunyankin, M.O.; Shin, J.E.; Zasadzinski, J.A. Near-Infrared Light Triggered-Release in Deep Brain Regions Using Ultra-photosensitive Nanovesicles. Angew. Chem. 2020, 132, 8686–8693. [Google Scholar] [CrossRef]
- el Founi, M.; Laroui, H.; Canup, B.S.B.; Ametepe, J.S.; Vanderesse, R.; Acherar, S.; Babin, J.; Ferji, K.; Chevalot, I.; Six, J.-L. Doxorubicin Intracellular Release Via External UV Irradiation of Dextran-g-Poly (o-Nitrobenzyl Acrylate) Photosensitive Nanoparticles. ACS Appl. Biomed. Mater. 2021, 4, 2742–2751. [Google Scholar] [CrossRef]
- Rapp, T.L.; DeForest, C.A. Targeting Drug Delivery with Light: A Highly Focused Approach. Adv. Drug Deliv. Rev. 2021, 171, 94–107. [Google Scholar] [CrossRef]
- Gebeyehu, B.T.; Lee, A.-W.; Huang, S.-Y.; Muhabie, A.A.; Lai, J.-Y.; Lee, D.-J.; Cheng, C.-C. Highly Stable Photosensitive Supramolecular Micelles for Tunable, Efficient Controlled Drug Release. Eur. Polym. J. 2019, 110, 403–412. [Google Scholar] [CrossRef]
- Beauté, L.; McClenaghan, N.; Lecommandoux, S. Photo-Triggered Polymer Nanomedicines: From Molecular Mechanisms to Therapeutic Applications. Adv. Drug Deliv. Rev. 2019, 138, 148–166. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Wang, X. Photoresponsive Submicron-Sized Hollow Spheres Obtained from Amphiphilic Azobenzene-Containing Random Copolymer. Polymer 2012, 53, 3975–3985. [Google Scholar] [CrossRef]
- Zhao, X.; Fang, X.; Yang, S.; Zhang, S.; Yu, G.; Liu, Y.; Zhou, Y.; Feng, Y.; Li, J. Light-Tuning Amphiphility of Host-Guest Alginate-Based Supramolecular Assemblies for Photo-Responsive Pickering Emulsions. Carbohydr. Polym. 2021, 251, 117072. [Google Scholar] [CrossRef]
- Li, P.; Zhang, J.; Dong, C.-M. Photosensitive Poly (o-Nitrobenzyloxycarbonyl-l-Lysine)-b-Peo Polypeptide Copolymers: Synthesis, Multiple Self-Assembly Behaviors, and the Photo/Ph-Thermo-Sensitive Hydrogels. Polym. Chem. 2017, 8, 7033–7043. [Google Scholar] [CrossRef]
- Wu, L.; Tuo, X.; Cheng, H.; Chen, Z.; Wang, X. Synthesis, Photoresponsive Behavior, and Self-Assembly of Poly (Acrylic Acid)-Based Azo Polyelectrolytes. Macromolecules 2001, 34, 8005–8013. [Google Scholar] [CrossRef]
- Wells, C.M.; Harris, M.; Choi, L.; Murali, V.P.; Guerra, F.D.; Jennings, J.A. Stimuli-Responsive Drug Release from Smart Polymers. J. Funct. Biomater. 2019, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Pang, X.; Liu, G. Near-Infrared Light-Triggered Polymeric Nanomicelles for Cancer Therapy and Imaging. ACS Biomater. Sci. Eng. 2017, 4, 1928–1941. [Google Scholar] [CrossRef]
- Liu, H.; Cui, S.W.; Chen, M.; Liang, R.; Xu, F. Protective Approaches and Mechanisms of Microencapsulation to the Survival of Probiotic Bacteria during Processing, Storage and Gastrointestinal Digestion: A Review. Crit. Rev. Food Sci. Nutr. 2017, 59, 8398. [Google Scholar] [CrossRef]
- Alsuraifi, A.; Curtis, A.; Lamprou, D.A.; Hoskins, C. Stimuli Responsive Polymeric Systems for Cancer Therapy. Pharmaceutics 2018, 10, 136. [Google Scholar] [CrossRef]
- Abdollahi, A.; Roghani-Mamaqani, H.; Razavi, B.; Salami-Kalajahi, M. The Light-Controlling of Temperature-Responsivity in Stimuli-Responsive Polymers. Polym. Chem. 2019, 10, 5686–5720. [Google Scholar] [CrossRef]
- Li, J.; Lee, W.Y.-W.; Wu, T.; Xu, J.; Zhang, K.; Wong, D.S.H.; Li, R.; Li, G.; Bian, L. Near-Infrared Light-Triggered Release of Small Molecules for Controlled Differentiation and Long-Term Tracking of Stem Cells in Vivo Using Upconversion Nanoparticles. Biomaterials 2016, 110, 1–10. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, P.; Li, Q.; Al-Khalaf, A.A.; Hozzein, W.N.; Zhang, F.; Li, X.; Zhao, D. Near-Infrared Triggered Decomposition of Nanocapsules with High Tumor Accumulation and Stimuli Responsive Fast Elimination. Angew. Chem. 2018, 130, 2641–2645. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, R.; Yang, H.; Bao, C.; Fan, J.; Wang, C.; Lin, Q.; Zhu, L. Light-Responsive Polymersomes with a Charge-Switch for Targeted Drug Delivery. J. Mater. Chem. B 2020, 8, 727–735. [Google Scholar] [CrossRef]
- An, X.; Gui, R. Stimuli-responsive liposome and control release drug. In Nanostructures for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 887–917. [Google Scholar]
- Alwattar, J.K.; Mneimneh, A.T.; Abla, K.K.; Mehanna, M.M.; Allam, A.N. Smart Stimuli-Responsive Liposomal Nanohybrid Systems: A Critical Review of Theranostic Behavior in Cancer. Pharmaceutics 2021, 13, 355. [Google Scholar] [CrossRef]
- Chen, W.; Goldys, E.M.; Deng, W. Light-Induced Liposomes for Cancer Therapeutics. Prog. Lipid Res. 2020, 79, 101052. [Google Scholar] [CrossRef]
- Liu, D.; Wang, S.; Xu, S.; Liu, H. Photocontrollable Intermittent Release of Doxorubicin Hydrochloride from Liposomes Embedded by Azobenzene-Contained Glycolipid. Langmuir 2017, 33, 1004–1012. [Google Scholar] [CrossRef]
- Li, Y.; Chakraborty, A.; Chen, J.; Xu, Q. Combinatorial Library of Light-Cleavable Lipidoid Nanoparticles for Intracellular Drug Delivery. ACS Biomater. Sci. Eng. 2019, 5, 2391–2398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, B.; Huang, S.; Cai, F.; Wang, G.; Yu, H. UV–Vis–NIR Light-Induced Bending of Shape-Memory Polyurethane Composites Doped with Azobenzene and Upconversion Nanoparticles. Polymer 2019, 178, 121644. [Google Scholar] [CrossRef]
- Zhao, C.; Tong, Y.; Li, X.; Shao, L.; Chen, L.; Lu, J.; Deng, X.; Wang, X.; Wu, Y. Photosensitive Nanoparticles Combining Vascular-Independent Intratumor Distribution and On-Demand Oxygen-Depot Delivery for Enhanced Cancer Photodynamic Therapy. Small 2018, 14, 1703045. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, G.; Hu, J.; Liu, S. Photo-and Reduction-Responsive Polymersomes for Programmed Release of Small and Macromolecular Payloads. Biomacromolecules 2018, 19, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Castiglione, K. Light-Driven Biocatalysis in Liposomes and Polymersomes: Where Are We Now? Catalysts 2019, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.; Hu, P.; Peng, H.; Zhang, N.; Zheng, Q.; He, Y. Near-Infrared Laser-Triggered, Self-Immolative Smart Polymersomes for in Vivo Cancer Therapy. Int. J. Nanomed. 2020, 15, 137. [Google Scholar] [CrossRef] [Green Version]
- Mousavizadeh, A.; Jabbari, A.; Akrami, M.; Bardania, H. Cell Targeting Peptides as Smart Ligands for Targeting of Therapeutic or Diagnostic Agents: A Systematic Review. Colloids Surf. B Biointerfaces 2017, 158, 507–517. [Google Scholar] [CrossRef]
- Molavipordanjani, S.; Hosseinimehr, S.J. Strategies for Conjugation of Biomolecules to Nanoparticles as Tumor Targeting Agents. Curr. Pharm. Des. 2019, 25, 3917–3926. [Google Scholar] [CrossRef]
- Majumder, J.; Taratula, O.; Minko, T. Nanocarrier-Based Systems for Targeted and Site Specific Therapeutic Delivery. Adv. Drug Deliv. Rev. 2019, 144, 57–77. [Google Scholar] [CrossRef]
- Wei, G.; Wang, Y.; Huang, X.; Hou, H.; Zhou, S. Peptide-based Nanocarriers for Cancer Therapy. Small Methods 2018, 2, 1700358. [Google Scholar] [CrossRef]
- Stefaniak, K.R.; Epley, C.C.; Novak, J.J.; McAndrew, M.L.; Cornell, H.D.; Zhu, J.; McDaniel, D.K.; Davis, J.L.; Allen, I.C.; Morris, A.J. Photo-Triggered Release of 5-Fluorouracil from a MOF Drug Delivery Vehicle. Chem. Commun. 2018, 54, 7617–7620. [Google Scholar] [CrossRef]
- Arrue, L.; Ratjen, L. Internal Targeting and External Control: Phototriggered Targeting in Nanomedicine. ChemMedChem 2017, 12, 1908–1916. [Google Scholar] [CrossRef] [Green Version]
- Dai, Q.; Zhao, H.; Cao, H.; Yang, J.; Zhao, W.; Wang, T.; Liu, L.; Fan, Z.; Wang, G. Photo-Triggered Conversion of Hydrophilic Fluorescent Biomimetic Nanostructures for Cell Imaging. Chem. Commun. 2019, 55, 596–599. [Google Scholar] [CrossRef]
- Yi, C.; Yu, Z.; Ren, Q.; Liu, X.; Wang, Y.; Sun, X.; Yin, S.; Pan, J.; Huang, X. Nanoscale ZnO-Based Photosensitizers for Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2020, 30, 101694. [Google Scholar] [CrossRef]
- Ruskowitz, E.R.; DeForest, C.A. Photoresponsive Biomaterials for Targeted Drug Delivery and 4D Cell Culture. Nat. Rev. Mater. 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Borah, B.M.; Cacaccio, J.; Watson, R.; Pandey, R.K. Phototriggered Release of Tumor-Imaging and Therapy Agents from Lyophilized Multifunctional Polyacrylamide Nanoparticles. ACS Appl. Biomed. Mater. 2019, 2, 5663–5675. [Google Scholar] [CrossRef]
- Kebebe, D.; Liu, Y.; Wu, Y.; Vilakhamxay, M.; Liu, Z.; Li, J. Tumor-Targeting Delivery of Herb-Based Drugs with Cell-Penetrating/Tumor-Targeting Peptide-Modified Nanocarriers. Int. J. Nanomed. 2018, 13, 1425. [Google Scholar] [CrossRef] [Green Version]
- Adamo, G.; Campora, S.; Ghersi, G. Functionalization of nanoparticles in specific targeting and mechanism release. In Nanostructures for Novel Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 57–80. [Google Scholar]
- Fernández, M.; Orozco, J. Advances in Functionalized Photosensitive Polymeric Nanocarriers. Polymers 2021, 13, 2464. [Google Scholar] [CrossRef]
- Hong, G.; Zou, Y.; Antaris, A.L.; Diao, S.; Wu, D.; Cheng, K.; Zhang, X.; Chen, C.; Liu, B.; He, Y. Ultrafast Fluorescence Imaging in Vivo with Conjugated Polymer Fluorophores in the Second Near-Infrared Window. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Wan, Z.; Jiang, H.; Xiao, X.; Feng, Q.; Meng, Y.; Yu, Y. Dimer Targeting Peptide Mediated Precise and Controllable Drug Delivery by Upconversion Nanocarriers for Breast Cancer Therapy. Mater. Des. 2021, 203, 109597. [Google Scholar] [CrossRef]
- Jadia, R.; Kydd, J.; Rai, P. Remotely Phototriggered, Transferrin-Targeted Polymeric Nanoparticles for the Treatment of Breast Cancer. Photochem. Photobiol. 2018, 94, 765–774. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Xie, X.; Wang, Z.; Gong, W.; Zhang, H.; Li, Y.; Yu, F.; Li, Z.; Mei, X. Dual-Modified Liposomes with a Two-Photon-Sensitive Cell Penetrating Peptide and NGR Ligand for SiRNA Targeting Delivery. Biomaterials 2015, 48, 84–96. [Google Scholar] [CrossRef]
- Cao, Z.; Li, D.; Wang, J.; Xiong, M.; Yang, X. Direct Nucleus-targeted Drug Delivery Using Cascade PHe/Photo Dual-sensitive Polymeric Nanocarrier for Cancer Therapy. Small 2019, 15, 1902022. [Google Scholar] [CrossRef]
- Banerjee, A.K.; Dickens, J.M. Dynamics of an Arbitrary Flexible Body in Large Rotation and Translation. J. Guid. Control Dyn. 1990, 13, 221–227. [Google Scholar] [CrossRef]
- Case, K.M. Velocity Fluctuations of a Body in a Fluid. Phys. Fluids 1971, 14, 2091–2095. [Google Scholar] [CrossRef]
- Jain, M.C. Textbook of Engineering Physics: Pt. i; Phi Learning: New Delhi, India, 2009; ISBN 8120338626. [Google Scholar]
- Synge, J.L. Principles of Mechanics; Read Books Ltd.: Seattle, WA, USA, 2011; ISBN 144654561X. [Google Scholar]
- Wen, Y.; Liu, Q.; Liu, Y. Temperature Gradient-Driven Motion and Assembly of Two-Dimensional (2D) Materials on the Liquid Surface: A Theoretical Framework and Molecular Dynamics Simulation. Phys. Chem. Chem. Phys. 2020, 22, 24097–24108. [Google Scholar] [CrossRef] [PubMed]
- Vinze, P.M.; Choudhary, A.; Pushpavanam, S. Motion of an Active Particle in a Linear Concentration Gradient. Phys. Fluids 2021, 33, 32011. [Google Scholar] [CrossRef]
- Zhang, X.; Müller, J.; Xia, J.; Garst, M.; Liu, X.; Zhou, Y. Motion of Skyrmions in Nanowires Driven by Magnonic Momentum-Transfer Forces. New J. Phys. 2017, 19, 65001. [Google Scholar] [CrossRef]
- Maric, T.; Nasir, M.Z.M.; Webster, R.D.; Pumera, M. Tailoring Metal/TiO2 Interface to Influence Motion of Light-Activated Janus Micromotors. Adv. Funct. Mater. 2020, 30, 1908614. [Google Scholar] [CrossRef]
- Vizsnyiczai, G.; Frangipane, G.; Maggi, C.; Saglimbeni, F.; Bianchi, S.; di Leonardo, R. Light Controlled 3D Micromotors Powered by Bacteria. Nat. Commun. 2017, 8, 1–7. [Google Scholar] [CrossRef]
- Maria Hormigos, R.; Jurado Sanchez, B.; Escarpa, A. Multi-Light-Responsive Quantum Dot Sensitized Hybrid Micromotors with Dual-Mode Propulsion. Angew. Chem. 2019, 131, 3160–3164. [Google Scholar] [CrossRef]
- Xia, D.; Su, K.; Wu, J.; Ding, Z. Influence of Solids Motion on Ultrasonic Horn Tip Erosion in Solid–Liquid Two-Phase Flows. Wear 2021, 480, 203928. [Google Scholar] [CrossRef]
- Jiao, J.; Xu, D.; Liu, Y.; Zhao, W.; Zhang, J.; Zheng, T.; Feng, H.; Ma, X. Mini-Emulsionfabricated Magnetic and Fluorescent Hybrid Janus Micro-Motors. Micromachines 2018, 9, 83. [Google Scholar] [CrossRef] [Green Version]
- Guix, M.; Weiz, S.M.; Schmidt, O.G.; Medina-sánchez, M. Self-Propelled Micro/Nanoparticle Motors. Part. Part. Syst. Charact. 2018, 35, 1700382. [Google Scholar] [CrossRef]
- Xu, X.; Huo, Z.; Guo, J.; Liu, H.; Qi, X.; Wu, Z. Micromotor-Derived Composites for Biomedicine Delivery and Other Related Purposes. Bio-Des. Manuf. 2020, 3, 133–147. [Google Scholar] [CrossRef]
- Wu, Z.; Lin, X.; Zou, X.; Sun, J.; He, Q. Biodegradable Protein-Based Rockets for Drug Transportation and Light-Triggered Release. ACS Appl. Mater. Interfaces 2015, 7, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wang, T.; Zheng, M.; Jiang, Z.; Wang, S. Controlled One-Sided Growth of Janus TiO2/MnO2 Nanomotors. Nanotechnology 2019, 30, 315702. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Nawar, S.; Werner, J.G.; Liu, J.; Huang, G.; Mei, Y.; Weitz, D.A.; Solovev, A.A. Hydrogel Micromotors with Catalyst-Containing Liquid Core and Shell. J. Phys. Condens. Matter 2019, 31, 214004. [Google Scholar] [CrossRef]
- Xuan, M.; Wu, Z.; Shao, J.; Dai, L.; Si, T.; He, Q. Near Infrared Light-Powered Janus Mesoporous Silica Nanoparticle Motors. J. Am. Chem. Soc. 2016, 138, 6492–6497. [Google Scholar] [CrossRef] [PubMed]
- Si, T.; Zou, X.; Wu, Z.; Li, T.; Wang, X.; Ivanovich, K.I.; He, Q. A Bubble-Dragged Catalytic Polymer Microrocket. Chem. Asian J. 2019, 14, 2460–2464. [Google Scholar] [CrossRef]
- Esteban-Fernández de Ávila, B.; Lopez-Ramirez, M.A.; Mundaca-Uribe, R.; Wei, X.; Ramírez-Herrera, D.E.; Karshalev, E.; Nguyen, B.; Fang, R.H.; Zhang, L.; Wang, J. Multicompartment Tubular Micromotors toward Enhanced Localized Active Delivery. Adv. Mater. 2020, 32, 2000091. [Google Scholar] [CrossRef]
- Schwarz, L.; Karnaushenko, D.D.; Hebenstreit, F.; Naumann, R.; Schmidt, O.G.; Medina-Sánchez, M. A Rotating Spiral Micromotor for Noninvasive Zygote Transfer. Adv. Sci. 2020, 7, 2000843. [Google Scholar] [CrossRef]
- Yánez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Janus Particles for (Bio) Sensing. Appl. Mater. Today 2017, 9, 276–288. [Google Scholar] [CrossRef]
- Wei, X.; Beltrán-Gastélum, M.; Karshalev, E.; Esteban-Fernández de Ávila, B.; Zhou, J.; Ran, D.; Angsantikul, P.; Fang, R.H.; Wang, J.; Zhang, L. Biomimetic Micromotor Enables Active Delivery of Antigens for Oral Vaccination. Nano Lett. 2019, 19, 1914–1921. [Google Scholar] [CrossRef]
- Xu, X.; Hou, S.; Wattanatorn, N.; Wang, F.; Yang, Q.; Zhao, C.; Yu, X.; Tseng, H.-R.; Jonas, S.J.; Weiss, P.S. Precision-Guided Nanospears for Targeted and High-Throughput Intracellular Gene Delivery. ACS Nano 2018, 12, 4503–4511. [Google Scholar] [CrossRef]
- Hansen-Bruhn, M.; de Ávila, B.E.; Beltrán-Gastélum, M.; Zhao, J.; Ramírez-Herrera, D.E.; Angsantikul, P.; Vesterager Gothelf, K.; Zhang, L.; Wang, J. Active Intracellular Delivery of a Cas9/SgRNA Complex Using Ultrasound-Propelled Nanomotors. Angew. Chem. Int. Ed. 2018, 57, 2657–2661. [Google Scholar] [CrossRef]
- Peifeng, S.; Hongxin, W.; Yongming, C.; Fei, P. Micro/Nanomotors as Drug Delivery Agent. Prog. Chem. 2019, 31, 63. [Google Scholar]
- Xu, D.; Zhou, C.; Zhan, C.; Wang, Y.; You, Y.; Pan, X.; Jiao, J.; Zhang, R.; Dong, Z.; Wang, W. Enzymatic Micromotors as a Mobile Photosensitizer Platform for Highly Efficient On-Chip Targeted Antibacteria Photodynamic Therapy. Adv. Funct. Mater. 2019, 29, 1807727. [Google Scholar] [CrossRef]
- Orozco, J.; Mercante, L.A.; Pol, R.; Merkoçi, A. Graphene-Based Janus Micromotors for the Dynamic Removal of Pollutants. J. Mater. Chem. A 2016, 4, 3371–3378. [Google Scholar] [CrossRef]
- Chi, Q.; Wang, Z.; Tian, F.; You, J.; Xu, S. A Review of Fast Bubble-Driven Micromotors Powered by Biocompatible Fuel: Low-Concentration Fuel, Bioactive Fluid and Enzyme. Micromachines 2018, 9, 537. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; de Avila, B.E.-F.; Zhang, L.; Wang, J. Targeting and Isolation of Cancer Cells Using Micro/Nanomotors. Adv. Drug Deliv. Rev. 2018, 125, 94–101. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y.; Ye, Z.; Lin, Z.; Ma, X.; He, Q. Biomedical Micro-/Nanomotors: From Overcoming Biological Barriers to In Vivo Imaging. Adv. Mater. 2021, 33, 2000512. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, C.; Wu, J.; Ju, H. Bubble-Propelled Jellyfish-like Micromotors for DNA Sensing. ACS Appl. Mater. Interfaces 2019, 11, 13581–13588. [Google Scholar] [CrossRef]
- Safdar, M.; Simmchen, J.; Jänis, J. Light-Driven Micro-and Nanomotors for Environmental Remediation. Environ. Sci. Nano 2017, 4, 1602–1616. [Google Scholar] [CrossRef]
- Jiang, W.; Ma, L.; Xu, X. Recent Progress on the Design and Fabrication of Micromotors and Their Biomedical Applications. Bio-Des. Manuf. 2018, 1, 225–236. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Song, B.; Zhang, H.; Duan, R.; Zhang, D.; Dong, B. A Light-Driven Micromotor with Complex Motion Behaviors for Controlled Release. Adv. Mater. Interfaces 2019, 6, 1801965. [Google Scholar] [CrossRef]
- Pal, M.; Somalwar, N.; Singh, A.; Bhat, R.; Eswarappa, S.M.; Saini, D.K.; Ghosh, A. Maneuverability of Magnetic Nanomotors inside Living Cells. Adv. Mater. 2018, 30, 1800429. [Google Scholar] [CrossRef]
- Santiago, I.; Jiang, L.; Foord, J.; Turberfield, A.J. Self-Propulsion of Catalytic Nanomotors Synthesised by Seeded Growth of Asymmetric Platinum–Gold Nanoparticles. Chem. Commun. 2018, 54, 1901–1904. [Google Scholar] [CrossRef]
- Hortelao, A.C.; Simó, C.; Guix, M.; Guallar-Garrido, S.; Julián, E.; Vilela, D.; Rejc, L.; Ramos-Cabrer, P.; Cossío, U.; Gómez-Vallejo, V. Swarming Behavior and in Vivo Monitoring of Enzymatic Nanomotors within the Bladder. Sci. Robot. 2021, 6, eabd2823. [Google Scholar] [CrossRef]
- Zhou, D.; Ren, L.; Li, Y.C.; Xu, P.; Gao, Y.; Zhang, G.; Wang, W.; Mallouk, T.E.; Li, L. Visible Light-Driven, Magnetically Steerable Gold/Iron Oxide Nanomotors. Chem. Commun. 2017, 53, 11465–11468. [Google Scholar] [CrossRef]
- Xing, Y.; Zhou, M.; Xu, T.; Tang, S.; Fu, Y.; Du, X.; Su, L.; Wen, Y.; Zhang, X.; Ma, T. Core@ Satellite Janus Nanomotors with PH-Responsive Multi-phoretic Propulsion. Angew. Chem. 2020, 132, 14474–14478. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, Y.; Qiu, X.; Zhang, T.; Chen, Q.; Huang, X. Marangoni Effect-Driven Motion of Miniature Robots and Generation of Electricity on Water. Langmuir 2017, 33, 12609–12615. [Google Scholar] [CrossRef]
- Fernández-Medina, M.; Ramos-Docampo, M.A.; Hovorka, O.; Salgueiriño, V.; Städler, B. Recent Advances in Nano-and Micromotors. Adv. Funct. Mater. 2020, 30, 1908283. [Google Scholar] [CrossRef]
- Pourrahimi, A.M.; Pumera, M. Multifunctional and Self-Propelled Spherical Janus Nano/Micromotors: Recent Advances. Nanoscale 2018, 10, 16398–16415. [Google Scholar] [CrossRef]
- Burelbach, J.; Frenkel, D.; Pagonabarraga, I.; Eiser, E. A Unified Description of Colloidal Thermophoresis. arXiv 2017, arXiv:1708.02195. [Google Scholar] [CrossRef] [Green Version]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [Green Version]
- Halder, A.; Sun, Y. Biocompatible Propulsion for Biomedical Micro/Nano Robotics. Biosens. Bioelectron. 2019, 139, 111334. [Google Scholar] [CrossRef]
- Villa, K.; Pumera, M. Fuel-Free Light-Driven Micro/Nanomachines: Artificial Active Matter Mimicking Nature. Chem. Soc. Rev. 2019, 48, 4966–4978. [Google Scholar] [CrossRef]
- Zhou, D.; Zhuang, R.; Chang, X.; Li, L. Enhanced Light-Harvesting Efficiency and Adaptation: A Review on Visible-Light-Driven Micro/Nanomotors. Research 2020, 2020, 6821595. [Google Scholar] [CrossRef]
- Xu, L.; Mou, F.; Gong, H.; Luo, M.; Guan, J. Light-Driven Micro/Nanomotors: From Fundamentals to Applications. Chem. Soc. Rev. 2017, 46, 6905–6926. [Google Scholar] [CrossRef]
- Wan, M.; Wang, Q.; Li, X.; Xu, B.; Fang, D.; Li, T.; Yu, Y.; Fang, L.; Wang, Y.; Wang, M. Systematic Research and Evaluation Models of Nanomotors for Cancer Combined Therapy. Angew. Chem. Int. Ed. 2020, 59, 14458–14465. [Google Scholar] [CrossRef]
- Wu, Y.; Si, T.; Shao, J.; Wu, Z.; He, Q. Near-Infrared Light-Driven Janus Capsule Motors: Fabrication, Propulsion, and Simulation. Nano Res. 2016, 9, 3747–3756. [Google Scholar] [CrossRef]
- Wu, Z.; Si, T.; Gao, W.; Lin, X.; Wang, J.; He, Q. Superfast Near-Infrared Light-Driven Polymer Multilayer Rockets. Small 2016, 12, 577–582. [Google Scholar] [CrossRef]
- Macintyre, S.A. Magnetic field measurement. In Measurement, Instrumentation, and Sensors Handbook; CRC Press: Boca Raton, FL, USA, 2017; pp. 31–39. ISBN 1315217449. [Google Scholar]
- Breton, A.; Soupault, P. The Magnetic Fields; New York Review of Books: New York, NY, USA, 2020; ISBN 1681374617. [Google Scholar]
- Xu, H.; Medina-Sánchez, M.; Schmidt, O.G. Magnetic Micromotors for Multiple Motile Sperm Cells Capture, Transport, and Enzymatic Release. Angew. Chem. Int. Ed. 2020, 59, 15029–15037. [Google Scholar] [CrossRef]
- Sun, M.; Fan, X.; Meng, X.; Song, J.; Chen, W.; Sun, L.; Xie, H. Magnetic Biohybrid Micromotors with High Maneuverability for Efficient Drug Loading and Targeted Drug Delivery. Nanoscale 2019, 11, 18382–18392. [Google Scholar] [CrossRef]
- Pourrahimi, A.M.; Villa, K.; Manzanares Palenzuela, C.L.; Ying, Y.; Sofer, Z.; Pumera, M. Catalytic and Light-Driven ZnO/Pt Janus Nano/Micromotors: Switching of Motion Mechanism via Interface Roughness and Defect Tailoring at the Nanoscale. Adv. Funct. Mater. 2019, 29, 1808678. [Google Scholar] [CrossRef]
- Hwang, J.; Yang, H.-M.; Lee, K.-W.; Jung, Y.-I.; Lee, K.J.; Park, C.W. A Remotely Steerable Janus Micromotor Adsorbent for the Active Remediation of Cs-Contaminated Water. J. Hazard. Mater. 2019, 369, 416–422. [Google Scholar] [CrossRef]
- Peng, F.; Men, Y.; Tu, Y.; Chen, Y.; Wilson, D.A. Nanomotor-Based Strategy for Enhanced Penetration across Vasculature Model. Adv. Funct. Mater. 2018, 28, 1706117. [Google Scholar] [CrossRef]
- Lu, A.X.; Liu, Y.; Oh, H.; Gargava, A.; Kendall, E.; Nie, Z.; DeVoe, D.L.; Raghavan, S.R. Catalytic Propulsion and Magnetic Steering of Soft, Patchy Microcapsules: Ability to Pick-up and Drop-off Microscale Cargo. ACS Appl. Mater. Interfaces 2016, 8, 15676–15683. [Google Scholar] [CrossRef]
- de Ávila, B.E.-F.; Angsantikul, P.; Li, J.; Lopez-Ramirez, M.A.; Ramírez-Herrera, D.E.; Thamphiwatana, S.; Chen, C.; Delezuk, J.; Samakapiruk, R.; Ramez, V. Micromotor-Enabled Active Drug Delivery for in Vivo Treatment of Stomach Infection. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, Y.; Peng, F.; White, P.B.; Wilson, D.A. Redox-sensitive Stomatocyte Nanomotors: Destruction and Drug Release in the Presence of Glutathione. Angew. Chem. Int. Ed. 2017, 56, 7620–7624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toebes, B.J.; Abdelmohsen, L.K.E.A.; Wilson, D.A. Enzyme-Driven Biodegradable Nanomotor Based on Tubular-Shaped Polymeric Vesicles. Polym. Chem. 2018, 9, 3190–3194. [Google Scholar] [CrossRef]
- Li, J.; Ji, F.; Ng, D.H.L.; Liu, J.; Bing, X.; Wang, P. Bioinspired Pt-Free Molecularly Imprinted Hydrogel-Based Magnetic Janus Micromotors for Temperature-Responsive Recognition and Adsorption of Erythromycin in Water. Chem. Eng. J. 2019, 369, 611–620. [Google Scholar] [CrossRef]
- Ma, X.; Hortelão, A.C.; Patino, T.; Sanchez, S. Enzyme Catalysis to Power Micro/Nanomachines. ACS Nano 2016, 10, 9111–9122. [Google Scholar] [CrossRef] [Green Version]
- Butler, P.J.; Dey, K.K.; Sen, A. Impulsive Enzymes: A New Force in Mechanobiology. Cell. Mol. Bioeng. 2015, 8, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Keller, S.; Teora, S.P.; Hu, G.X.; Nijemeisland, M.; Wilson, D.A. High-Throughput Design of Biocompatible Enzyme-Based Hydrogel Microparticles with Autonomous Movement. Angew. Chem. 2018, 130, 9962–9965. [Google Scholar] [CrossRef]
- Chen, H.; Li, W.; Lin, Y.; Wang, L.; Liu, X.; Huang, X. Fusion-Induced Structural and Functional Evolution in Binary Emulsion Communities. Angew. Chem. 2020, 132, 17101–17108. [Google Scholar] [CrossRef]
- Wang, L.; Lin, Y.; Zhou, Y.; Xie, H.; Song, J.; Li, M.; Huang, Y.; Huang, X.; Mann, S. Autonomic Behaviors in Lipase-Active Oil Droplets. Angew. Chem. 2019, 131, 1079–1083. [Google Scholar] [CrossRef]
- Wong, F.; Sen, A. Progress toward Light-Harvesting Self-Electrophoretic Motors: Highly Efficient Bimetallic Nanomotors and Micropumps in Halogen Media. ACS Nano 2016, 10, 7172–7179. [Google Scholar] [CrossRef]
- Wang, W.; Chiang, T.-Y.; Velegol, D.; Mallouk, T.E. Understanding the Efficiency of Autonomous Nano-and Microscale Motors. J. Am. Chem. Soc. 2013, 135, 10557–10565. [Google Scholar] [CrossRef]
- Xiao, Z.; Wei, M.; Wang, W. A Review of Micromotors in Confinements: Pores, Channels, Grooves, Steps, Interfaces, Chains, and Swimming in the Bulk. ACS Appl. Mater. Interfaces 2018, 11, 6667–6684. [Google Scholar] [CrossRef]
- Popescu, M.N.; Uspal, W.E.; Dietrich, S. Self-Diffusiophoresis of Chemically Active Colloids. Eur. Phys. J. Spec. Top. 2016, 225, 2189–2206. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Zhang, H.P.; Tang, J.; Wang, W. Photochemically Powered AgCl Janus Micromotors as a Model System to Understand Ionic Self-Diffusiophoresis. Langmuir 2018, 34, 3289–3295. [Google Scholar] [CrossRef]
- Manjare, M.; Yang, F.; Qiao, R.; Zhao, Y. Marangoni Flow Induced Collective Motion of Catalytic Micromotors. J. Phys. Chem. C 2015, 119, 28361–28367. [Google Scholar] [CrossRef]
- Orozco, J.; Vilela, D.; Valdés-Ramírez, G.; Fedorak, Y.; Escarpa, A.; Vazquez-Duhalt, R.; Wang, J. Efficient Biocatalytic Degradation of Pollutants by Enzyme-Releasing Self-Propelled Motors. Chem. -A Eur. J. 2014, 20, 2866–2871. [Google Scholar] [CrossRef]
- Gupta, S.; Zhang, Q.; Emrick, T.; Balazs, A.C.; Russell, T.P. Entropy-Driven Segregation of Nanoparticles to Cracks in Multilayered Composite Polymer Structures. Nat. Mater. 2006, 5, 229–233. [Google Scholar] [CrossRef]
- Chroni, A.; Mavromoustakos, T.; Pispas, S. Biocompatible PEO-b-PCL Nanosized Micelles as Drug Carriers: Structure and Drug–Polymer Interactions. Nanomaterials 2020, 10, 1872. [Google Scholar] [CrossRef]
- Liu, M.; Sun, Y.; Wang, T.; Ye, Z.; Zhang, H.; Dong, B.; Li, C.Y. A Biodegradable, All-Polymer Micromotor for Gas Sensing Applications. J. Mater. Chem. C 2016, 4, 5945–5952. [Google Scholar] [CrossRef]
- Gao, W.; Wang, J. Synthetic Micro/Nanomotors in Drug Delivery. Nanoscale 2014, 6, 10486–10494. [Google Scholar] [CrossRef] [Green Version]
- Salimi, A.; Zadeh, B.S.M.; Kazemi, M. Preparation and Optimization of Polymeric Micelles as an Oral Drug Delivery System for Deferoxamine Mesylate: In Vitro and Ex Vivo Studies. Res. Pharm. Sci. 2019, 14, 293. [Google Scholar]
- Prajapati, S.K.; Jain, A.; Jain, A.; Jain, S. Biodegradable Polymers and Constructs: A Novel Approach in Drug Delivery. Eur. Polym. J. 2019, 120, 109191. [Google Scholar] [CrossRef]
- Guo, Y.; di Mare, L.; Li, R.K.Y.; Wong, J.S.S. Cargo Release from Polymeric Vesicles under Shear. Polymers 2018, 10, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markwalter, C.E.; Pagels, R.F.; Hejazi, A.N.; Gordon, A.G.R.; Thompson, A.L.; Prud’homme, R.K. Polymeric Nanocarrier Formulations of Biologics Using Inverse Flash NanoPrecipitation. AAPS J. 2020, 22, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Peng, F.; Wilson, D.A. Motion Manipulation of Micro-and Nanomotors. Adv. Mater. 2017, 29, 1701970. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, B.; Zhao, Z.; Yang, X.; Xing, Y.; You, C.; Kong, Y.; Cui, J.; Zhu, L.; Lin, S. Flying Squirrel-Inspired Motion Control of a Light-Deformed Pt-PAzoMA Micromotor through Drag Force Manipulation. ACS Appl. Mater. Interfaces 2021, 13, 30106–30117. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, L.; Wei, Y.; El-Toni, A.M.; Zhang, F.; Zhao, D. Anisotropic Growth-Induced Synthesis of Dual-Compartment Janus Mesoporous Silica Nanoparticles for Bimodal Triggered Drugs Delivery. J. Am. Chem. Soc. 2014, 136, 15086–15092. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Sen, S.; Udayabhaskararao, T.; Sawczyk, M.; Kučanda, K.; Manna, D.; Kundu, P.K.; Lee, J.-W.; Král, P.; Klajn, R. Reversible Trapping and Reaction Acceleration within Dynamically Self-Assembling Nanoflasks. Nat. Nanotechnol. 2016, 11, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Bujalance-Fernández, J.; Jurado-Sánchez, B.; Escarpa, A. Light-Driven Nanomotors and Micromotors: Envisioning New Analytical Possibilities for Bio-Sensing. Microchim. Acta 2020, 187, 1–16. [Google Scholar] [CrossRef]
- Palagi, S.; Singh, D.P.; Fischer, P. Light-controlled Micromotors and Soft Microrobots. Adv. Opt. Mater. 2019, 7, 1900370. [Google Scholar] [CrossRef]
- Abid, J.-P.; Frigoli, M.; Pansu, R.; Szeftel, J.; Zyss, J.; Larpent, C.; Brasselet, S. Light-Driven Directed Motion of Azobenzene-Coated Polymer Nanoparticles in an Aqueous Medium. Langmuir 2011, 27, 7967–7971. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.; Qin, H.; Zhao, Z.; Liu, H. Light-Driven and Light-Guided Microswimmers. Adv. Funct. Mater. 2016, 26, 3164–3171. [Google Scholar] [CrossRef]
- Tezel, G.; Timur, S.S.; Kuralay, F.; Gürsoy, R.N.; Ulubayram, K.; Öner, L.; Eroğlu, H. Current Status of Micro/Nanomotors in Drug Delivery. J. Drug Target. 2021, 29, 29–45. [Google Scholar] [CrossRef]
- Agrahari, V.; Agrahari, V.; Chou, M.-L.; Chew, C.H.; Noll, J.; Burnouf, T. Intelligent Micro-/Nanorobots as Drug and Cell Carrier Devices for Biomedical Therapeutic Advancement: Promising Development Opportunities and Translational Challenges. Biomaterials 2020, 260, 120163. [Google Scholar] [CrossRef]
- Shao, J.; Cao, S.; Williams, D.S.; Abdelmohsen, L.K.E.A.; van Hest, J.C.M. Photoactivated Polymersome Nanomotors: Traversing Biological Barriers. Angew. Chem. Int. Ed. 2020, 59, 16918–16925. [Google Scholar] [CrossRef]
- Dong, R.; Hu, Y.; Wu, Y.; Gao, W.; Ren, B.; Wang, Q.; Cai, Y. Visible-Light-Driven BiOI-Based Janus Micromotor in Pure Water. J. Am. Chem. Soc. 2017, 139, 1722–1725. [Google Scholar] [CrossRef] [Green Version]
- Kochergin, Y.S.; Villa, K.; Novotný, F.; Plutnar, J.; Bojdys, M.J.; Pumera, M. Multifunctional Visible-Light Powered Micromotors Based on Semiconducting Sulfur-and Nitrogen-Containing Donor–Acceptor Polymer. Adv. Funct. Mater. 2020, 30, 2002701. [Google Scholar] [CrossRef]
- Jurado-Sánchez, B.; Pacheco, M.; Rojo, J.; Escarpa, A. Magnetocatalytic Graphene Quantum Dots Janus Micromotors for Bacterial Endotoxin Detection. Angew. Chem. Int. Ed. 2017, 56, 6957–6961. [Google Scholar] [CrossRef]
- Choi, H.; Lee, G.-H.; Kim, K.S.; Hahn, S.K. Light-Guided Nanomotor Systems for Autonomous Photothermal Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 2338–2346. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; He, J.; Hourwitz, M.J.; Yang, Y.; Fourkas, J.T.; Han, X.; Nie, Z. Continuous Microfluidic Self-Assembly of Hybrid Janus-like Vesicular Motors: Autonomous Propulsion and Controlled Release. Small 2015, 11, 3762–3767. [Google Scholar] [CrossRef]
- Bozuyuk, U.; Yasa, O.; Yasa, I.C.; Ceylan, H.; Kizilel, S.; Sitti, M. Light-Triggered Drug Release from 3D-Printed Magnetic Chitosan Microswimmers. ACS Nano 2018, 12, 9617–9625. [Google Scholar] [CrossRef]
- Ameta, R.; Solanki, M.S.; Benjamin, S.; Ameta, S.C. Photocatalysis. In Advanced Oxidation Processes for Waste Water Treatment; Elsevier: Amsterdam, The Netherlands, 2018; pp. 135–175. [Google Scholar]
- Khalid, N.R.; Majid, A.; Tahir, M.B.; Niaz, N.A.; Khalid, S. Carbonaceous-TiO2 Nanomaterials for Photocatalytic Degradation of Pollutants: A Review. Ceram. Int. 2017, 43, 14552–14571. [Google Scholar] [CrossRef]
- Tong, J.; Wang, D.; Wang, D.; Xu, F.; Duan, R.; Zhang, D.; Fan, J.; Dong, B. Visible-Light-Driven Water-Fueled Ecofriendly Micromotors Based on Iron Phthalocyanine for Highly Efficient Organic Pollutant Degradation. Langmuir 2019, 36, 6930–6937. [Google Scholar] [CrossRef]
- Mera, A.C.; Rodríguez, C.A.; Meléndrez, M.F.; Valdés, H. Synthesis and Characterization of BiOI Microspheres under Standardized Conditions. J. Mater. Sci. 2017, 52, 944–954. [Google Scholar] [CrossRef]
- Alenizi, M.A.; Kumar, R.; Aslam, M.; Alseroury, F.A.; Barakat, M.A. Construction of a Ternary GC3N4/TiO2@ Polyaniline Nanocomposite for the Enhanced Photocatalytic Activity under Solar Light. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Metkar, S.K.; Girigoswami, K. Diagnostic Biosensors in Medicine–A Review. Biocatal. Agric. Biotechnol. 2019, 17, 271–283. [Google Scholar] [CrossRef]
- Khalilzadeh, B.; Shadjou, N.; Kanberoglu, G.S.; Afsharan, H.; de La Guardia, M.; Charoudeh, H.N.; Ostadrahimi, A.; Rashidi, M.-R. Advances in Nanomaterial Based Optical Biosensing and Bioimaging of Apoptosis via Caspase-3 Activity: A Review. Microchim. Acta 2018, 185, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.-H.; Wu, K.-J.; Li, G.; Wu, C.; Ko, C.-N.; Ma, D.-L. Application of Label-Free Techniques in Microfluidic for Biomolecules Detection and Circulating Tumor Cells Analysis. TrAC Trends Anal. Chem. 2019, 117, 78–83. [Google Scholar] [CrossRef]
- Otero, F.; Santiso, R.; Tamayo, M.; Fernández, J.L.; Bou, G.; Lepe, J.A.; McConnell, M.J.; Gosálvez, J.; Cisneros, J.M. Rapid Detection of Antibiotic Resistance in Gram-Negative Bacteria through Assessment of Changes in Cellular Morphology. Microb. Drug Resist. 2017, 23, 157–162. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, C.; Lun, Z.; Lv, Y.; Chen, W.; Li, T. Crosstalk between P38 MAPK and Caspase-9 Regulates Mitochondria-Mediated Apoptosis Induced by Tetra-α-(4-Carboxyphenoxy) Phthalocyanine Zinc Photodynamic Therapy in LoVo Cells. Oncol. Rep. 2018, 39, 61–70. [Google Scholar]
- Staszak, K.; Wieszczycka, K.; Marturano, V.; Tylkowski, B. Lanthanides Complexes–Chiral Sensing of Biomolecules. Coord. Chem. Rev. 2019, 397, 76–90. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, H.; Wang, Z.; Song, D. Peptide-Functionalized Upconversion Nanoparticles-Based FRET Sensing Platform for Caspase-9 Activity Detection in Vitro and in Vivo. Biosens. Bioelectron. 2019, 141, 111403. [Google Scholar] [CrossRef]
- Jurado-Sánchez, B.; Escarpa, A. Janus Micromotors for Electrochemical Sensing and Biosensing Applications: A Review. Electroanalysis 2017, 29, 14–23. [Google Scholar] [CrossRef]
- Wang, S.; Xu, J.; Zhou, Q.; Geng, P.; Wang, B.; Zhou, Y.; Liu, K.; Peng, F.; Tu, Y. Biodegradability of Micro/Nanomotors: Challenges and Opportunities. Adv. Healthc. Mater. 2021, 10, 2100335. [Google Scholar] [CrossRef]
- Ray, M.; Lee, Y.-W.; Scaletti, F.; Yu, R.; Rotello, V.M. Intracellular Delivery of Proteins by Nanocarriers. Nanomedicine 2017, 12, 941–952. [Google Scholar] [CrossRef] [Green Version]
- Mathesh, M.; Sun, J.; Wilson, D.A. Enzyme Catalysis Powered Micro/Nanomotors for Biomedical Applications. J. Mater. Chem. B 2020, 8, 7319–7334. [Google Scholar] [CrossRef]







| Characterization Parameter | Method | Comment | Ref. |
|---|---|---|---|
| Morphological and physicochemical | FT-IR | The absorption spectroscopy technique works via application of a polychromatic infrared beam over a compound in liquid, solid, or gaseous state. | [159] |
| The infrared spectrum is obtained via the Fourier transform mathematical process, in a wavenumber range between 4000 cm−1 and 660 cm−1. | [160] | ||
| Chemical functional groups—such as carboxylic, hydroxy, and amine groups, among many others—absorb and transmit infrared light at different wavenumbers, characterizing the PMNs’ organic composition. | [161,162] | ||
| NMR | Structural characterization technology in which an oscillating magnetic field disturbs a nucleus molecule, producing a particular electromagnetic signal. | [163] | |
| The frequency of this signal depends on the chemical structure and nonzero nuclear spin of the involved isotope. | [164] | ||
| 1H NMR is a spectrometric method that uses the magnetic moment and angular momentum of the hydrogen-1 nucleus to obtain its frequency characteristics, which depend on the atom–proton interaction, such as C–H and O–H, determining the molecular structure of the PMNs. | [165,166] | ||
| TEM | The micro/nanoscale image capture method detects the transmitted electrons after irradiation with an electron beam over a target sample. | [167] | |
| The staining technique from TEM permits differentiation between nanopolymersomes, nanomicelles, and nanospheres, because the hydrophobic core and interface of the micelles and polymersomes are stained for differentiation via dark electron absorption (Figure 2B,C, respectively). | [168,169,170] | ||
| SEM | Electron-beam-based technique that scans the sample’s surface via electron–atom interaction, characterizing its topography and composition. | [171] | |
| The spheres with a high density of hydrophobic and hydrophilic segments can be visualized via SEM (Figure 4D). | |||
| Morphological and physicochemical | TEM and SEM | Characterizes morphology, size, and elemental composition of the PMNs. | [172] |
| DLS | Uses an optical mode to quantify the scattered light beam for the particles dispersed in the medium, characterizing the size distribution of PMNs, concerning the sample’s intensity extent, volume, or number. | [173,174] | |
| SLS | Estimates the molecular weight of NPs in the solution. | [175] | |
| ELS | Describes the static electric field intensity on the double-layer limit from a particle/molecule dispersed in a fluid. | [176] | |
| The superficial charge known as ζ-potential determines the PMNs’ dispersity and the electron density characteristics of the available groups on the nanoparticles’ surface. | [177] | ||
| Fluorescence spectroscopy | Quantifies a compound’s light emission after electron excitation via light beam irradiation. | [178] | |
| Absorbance spectroscopy | Spectrophotometric method characterized by wavelength-dependent absorption of a compound after light irradiation. | [179] | |
| Absorbance spectroscopy describes the photoisomerization and photocleavage mechanism by absorbing the PAMs’ characteristic wavelength peaks. | [154,156] | ||
| Fluorescence and absorbance spectroscopy | Allows tracking and quantification of the presence of fluorescence and absorbance of loaded and photoreleased cargo in the PMNs, such as Nile red, doxorubicin, Dil, etc. | [147,157,180] | |
| Cargo LC, EE, and photorelease | Gravimetry, titrimetry, and potentiometry | Employed to quantify the concentration of a compound photoreleased from PMNs. | [181] |
| Gravimetry | Quantitative measurement of the solid analyte weight to concentration determination. | [182] | |
| Titrimetry | The quantitative chemical analysis uses neutralization reactions to measure a solution’s concentration via volumetric or potentiometric techniques. | [183] | |
| Interaction between the solutions involved in the reactions and an indicator that generates a color change characterizes the volumetric techniques to calculate the volumes’ concentration. | [184] | ||
| Potentiometry | Employs the turning points of the pH–volume curve between the target and the known solution in order to determine the hydrogen cation concentration and, indirectly, the concentrations of some compounds. | [185] | |
| Cargo LC, EE, and photorelease | Chromatography | Methods characterized by a physical separation, employing the mobile and stationary phases of the molecules involved in the process in gas and liquid states to quantify their concentrationa. | [186,187] |
| High-performance liquid chromatography (HPLC) quantifies cargo concentration by separating mobile and stationary liquid phases in order to establish the LC and EE. | [188] | ||
| Spectrophotometry and fluorescence spectroscopy | Commonly used to quantify the cargo concentration via UV–Vis absorption and fluorescence intensity techniques, using the cargo absorbance or fluorescence curves regarding concentration and weight—either directly in the loaded cargo or indirectly in the supernatant (free cargo)—and then determining the LC and EE. | [189] | |
| Spectrophotometry is the most commonly used method to quantify the delivered cargo, because of its straightforward use, taking advantage of the cargo absorption and fluorescence properties, as characterized by UV–Vis absorption and fluorescence techniques. | [190,191] | ||
| Movement | Tracking analysis | Motion videos are taken by high-resolution cameras adapted to optical and fluorescence microscopy. They are then analyzed using tracking software or programs such as Image J to quantify the velocity and distance traveled by the particles. | [192,193,194] |
| Nanoparticle trace analysis (NTA) | Nanoparticles’ motion is analyzed in solution using NanoSight for particles between 10 and 1000 nm in size. Each particle is analyzed individually using NTA, via direct observation and measurement of diffusion events. | [195,196] |
| Type | PAMs | Features | Application | Ref. |
|---|---|---|---|---|
| Polymeric nanomicelles | Azobenzene | 30s/UV light/365 nm/photoisomerization | Concept of cargo photodelivery | [211] |
| 5 min/UV light/365 nm/photoisomerization | Hydrophobic model drug (Nile red) photorelease | [180] | ||
| UV light/365 nm/photoisomerization | Hydrophobic model drug (Curcumin) photorelease | [212] | ||
| 14 s/UV light/365 nm/photoisomerization | Concept of specific cargo photorelease into cells | [146] | ||
| O-nitrobenzyl | 30 min/UV light/365 nm/photocleavage | Drug/gene/protein photodelivery | [213] | |
| Polymeric nanomicelles | O-nitrobenzyl | 60 min/NIR irradiation/765nm/photocleavage | Drug model photorelease in deep tissues | [149] |
| Liposomes | Azobenzene | UV light/365 nm/photoisomerization | Doxorubicin delivery | [226] |
| O-nitrobenzyl | NIR irradiation photocleavage | Concept of cargo photodelivery | [227] | |
| NIR irradiation/740 nm/pcCPP-linked | siRNA photodelivery into specific cells | [249] | ||
| Phospholipid-PEG nanoparticles | Fluorophore model | NIR irradiation ≥ 1000 nm/anti-EGFR antibody-linked | NIR fluorescence in vivo imaging of MDA-MB-468 cells | [246] |
| Nanopolymersomes | Azobenzene | UV light/360 nm/photoisomerization | Photorelease of hydrophilic and hydrophobic molecules | [147] |
| O-nitrobenzyl | UV light/365 nm/photocleavage | Photorelease of hydrophobic doxorubicin -release as potential use in cancer therapy | [140] | |
| UV light/360 nm/photocleavage | Doxorubicin photodelivery into HeLa cells | [222] | ||
| Nanospheres based on UCNPs | O-nitrobenzyl | 60 min/NIR irradiation/980 nm/photocleavage | Model drug delivery into cells for enhancing neocartilage formation in vivo | [220] |
| NIR irradiation/980 nm/adamantane-dimer-targeting peptide-linked | Drug photodelivery into cancer cells | [247] | ||
| Polymeric nanospheres | Verteporfin photosensitizer | NIR irradiation/690 nm/hTfr peptide-linked | Phototriggered treatment of breast cancer | [248] |
| DTRCD | NIR irradiation/660 nm/ROS photogeneration | Specific drug delivery into cell nuclei and enhanced cancer therapy | [250] |
| Motor | Propulsion Mechanism | Features | Application | Ref. |
|---|---|---|---|---|
| Janus capsule based on silica particles and gold | Thermophoresis triggered by light | NIR irradiation/808 nm/1–20 μm diameter/42 μm s−1 speed | Dynamic strategy for cancer therapy | [301] |
| Polymeric tubular rocket | NIR irradiation/780 nm/10 μm length and 5 μm width/160 μm s−1 speed | Fuel-free and externally controlled propulsion for biomedical applications | [302] | |
| Polymeric Janus microcapsules | Magneto-catalytic reaction triggered by the Pt–H2O2 system | ~150 μm diameter/200–2400 μm s−1 speed | Potential usefulness of soft micromotors for targeted delivery of cargo | [310] |
| Janus polymeric sphere | Catalytic reaction from the Mg–HCl system | ~20 μm diameter/~120 μm s−1 speed | Antibiotic drug delivery for bacterial burden reduction in the murine stomach | [311] |
| Tubular polymersome | Catalytic reaction from the catalase–H2O2 system | 300 nm size | Hydrophobic and hydrophilic model drug delivery | [313] |
| Protein- and phospholipid-based Janus droplets | Hydrolysis reaction from the urease–urea and lipase system | 22–80 µm size/2–41 µm s−1 speed | New Janus particle formation via fusion mechanism | [318] |
| Self-fueled lipase-active oil droplets | Buoyancy mechanism motion | 10 of 60 µm of oil droplets/membrane thickness of 7.46 nm | New lipase droplet for protocell buoyancy-induced motion | [319] |
| Polymeric Janus stomatocyte | Catalytic reaction from the Pt–H2O2 system | 345 nm diameter/~35 μm s−1 speed | Cargo delivery in a cellular environment | [312] |
| Nanorods | Electrophoresis triggered by UV or visible light | 3 μm length and 150 nm radius/21 ± 4 μm s−1 speed | Propulsion concept | [323] |
| Janus Ag-Cl | Diffusiophoresis | 2.5 μm diameter | 3D cargo transport and sensing applications | [324] |
| Tubular | Marangoni effect by the surfactant at tubular surface | Enzyme delivery | Pollutant degradation | [326] |
| Polymeric sphere | Photoisomerization triggered by light | UV and visible irradiation/16 nm diameter/15 μm s−1 speed | Light-stimulated propulsion | [341] |
| Janus polymersome | Thermophoresis stimulated by NIR irradiation | 100 nm diameter/6.2 μm s−1 speed | Doxorubicin delivery into HeLa cancer cells | [345] |
| Janus polymersome | Catalytic reaction caused by Pt–H2O2 | 749.2–2634.2 nm diameter/2–90 μm s−1 speed | Drug photodelivery | [350] |
| Magnetic chitosan microswimmers | Magnetic motion at 10 mT and 4.5 Hz | 3.34 ± 0.71 μm s−1 | Doxorubicin photocleavage release | [351] |
| Janus BiOI microspheres | Electrophoresis stimulated by visible light | 2~4 μm diameter/1.62 μm s−1 speed | Fuel-free motion strategy | [346] |
| Organic polymeric systems | Photocatalysis of semiconductor polymers under visible light | 6.5 μm diameter/1.82 μm s−1 speed | Colorimetric-based acidity sensors | [347] |
| Polymeric Janus microparticle | Magneto-catalytic motion from the Pt–H2O2 system | Fluorescent phenylboronic acid and graphene-modified GQDs | Detection of lipopolysaccharides for biomedical and biological applications | [248] |
| Polymeric Janus nanoparticle | Catalytic reaction from the Pt–H2O2 system | NIR thermal polymer | Photothermal therapy | [349] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mena-Giraldo, P.; Orozco, J. Polymeric Micro/Nanocarriers and Motors for Cargo Transport and Phototriggered Delivery. Polymers 2021, 13, 3920. https://doi.org/10.3390/polym13223920
Mena-Giraldo P, Orozco J. Polymeric Micro/Nanocarriers and Motors for Cargo Transport and Phototriggered Delivery. Polymers. 2021; 13(22):3920. https://doi.org/10.3390/polym13223920
Chicago/Turabian StyleMena-Giraldo, Pedro, and Jahir Orozco. 2021. "Polymeric Micro/Nanocarriers and Motors for Cargo Transport and Phototriggered Delivery" Polymers 13, no. 22: 3920. https://doi.org/10.3390/polym13223920
APA StyleMena-Giraldo, P., & Orozco, J. (2021). Polymeric Micro/Nanocarriers and Motors for Cargo Transport and Phototriggered Delivery. Polymers, 13(22), 3920. https://doi.org/10.3390/polym13223920