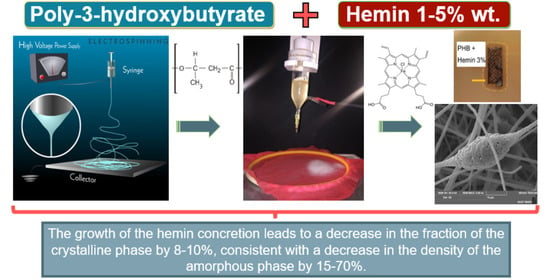
Effect of the Hemin Molecular Complexes on the Structure and Properties of the Composite Electrospun Materials Based on Poly(3-hydroxybutyrate)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Electrospun Materials
2.3. Methods
2.3.1. Microscopy
2.3.2. Morphology and Density Analysis
2.3.3. The Energy-Dispersive X-ray Spectroscopy
2.3.4. Differential Scanning Calorimetry
2.3.5. Electron Paramagnetic Resonance
2.3.6. Mechanical Properties
2.3.7. Antimicrobial Tests
3. Results
3.1. Morphology of the Fibrous Material
3.1.1. Results of the Optical Microscopy
3.1.2. Results of the Scanning Electron Microscopy
3.2. Chemical Constitution of the Material
Results of the EDX
3.3. Supramolecular Structure of the Material
3.3.1. Crystalline Phase
3.3.2. Amorphous Phase
3.4. Physical and Mechanical Properties
3.5. The Antimicrobial Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lund, A.; Velden, N.M.; Persson, N.K.; Hamedi, M.M.; Müller, C. Electrically conducting fibres for e-textiles: An open playground for conjugated polymers and carbon nanomaterials. Mater. Sci. Eng. R Rep. 2018, 126, 1–29. [Google Scholar] [CrossRef]
- Bailey, F.; Malinski, T.; Kiechle, F. Carbon-fiber ultramicroelectrodes modified with conductive polymeric tetrakis (3-methoxy-4-hydroxyphenyl) porphyrin for determination of nickel in single biological cells. Anal. Chem. 1991, 63, 395–398. [Google Scholar] [CrossRef]
- Avossa, J.; Paolesse, R.; Di Natale, C.; Zampetti, E.; Bertoni, G.; De Cesare, F.; Macagnano, A. Electrospinning of polystyrene/polyhydroxybutyrate nanofibers doped with porphyrin and graphene for chemiresistor gas sensors. Nanomaterials 2019, 9, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabassum, R.; Kant, R. Recent trends in surface plasmon resonance based fiber–optic gas sensors utilizing metal oxides and carbon nanomaterials as functional entities. Sens. Actuators B Chem. 2020, 310, 127813. [Google Scholar] [CrossRef]
- Scheicher, S.R.; Kainz, B.; Köstler, S.; Suppan, M.; Bizzarri, A.; Pum, D.; Ribitsch, V. Optical oxygen sensors based on Pt (II) porphyrin dye immobilized on S-layer protein matrices. Biosens. Bioelectron. 2009, 25, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Laskowska, M.; Kityk, L.; Pastukh, O.; Dulski, M.; Zubko, M.; Jedryka, J.; Laskowski, E. Nanocomposite for photonics—Nickel pyrophosphate nanocrystals synthesised in silica nanoreactors. Microporous Mesoporous Mater. 2020, 306, 110435. [Google Scholar] [CrossRef]
- Biswas, S.; Ahn, H.Y.; Bondar, M.V.; Belfield, K.D. Two-photon absorption enhancement of polymer-templated porphyrin-based J-aggregates. Langmuir 2011, 28, 1515–1522. [Google Scholar] [CrossRef]
- Chen, Z.; Mai, B.; Tan, H.X. The effect of thermally developed SiC and SiO2 core-shell structured nanoparticles on the mechanical, thermal and UV-shielding properties of polyimide composites. Comp. Comm. 2018, 10, 194–204. [Google Scholar] [CrossRef]
- Suo, Z.; Chen, J.; Hou, X.; Hu, Z.; Xing, F.; Feng, L. Growing prospects of DNA nanomaterials in novel biomedical applications. RSC Adv. 2019, 9, 16479–16491. [Google Scholar] [CrossRef] [Green Version]
- Ghosal, K.; Agatemor, C.; Špitálsky, Z.; Thomas, Z.; Kny, E. Electrospinning tissue engineering and wound dressing scaffolds from polymer–titanium dioxide nanocomposites. Chem. Eng. J. 2019, 358, 1262–1278. [Google Scholar] [CrossRef]
- Wu, J.; Li, S.; Wei, H. Integrated nanozymes: Facile preparation and biomedical applications. ChemComm 2018, 54, 6520–6530. [Google Scholar] [CrossRef]
- Ruthard, C.; Schmidt, M.; Gröhn, F. Porphyrin-polymer networks, worms, and nanorods: pH-triggerable hierarchical self-assembly. Macromol. Rapid Commun. 2011, 32, 706–711. [Google Scholar] [CrossRef]
- Almaguer-Flores, A.; Silva-Bermúdez, P.; Rodi, S.E. Nanostructured Biomaterials for Regenerative Medicine. In Nanostructured Biomaterials for Regenerative Medicine, 1st ed.; Guarino, V., Iafisco, M., Spriano, S., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 81–137. [Google Scholar]
- Chifiriuc, M.C.; Ficai, A.; Grumezescu, A.M.; Ditu, L.M.; Popa, M.; Iordache, C.; Lazar, V. Soft tissue engineering and microbial infections: Challenges and perspectives. In Nanobiomaterials in Soft Tissue Engineering; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 1–29. [Google Scholar]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Sessler, J.L.; Tomat, E. Transition-metal complexes of expanded porphyrins. Acc. Chem. Res. 2007, 40, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Sessler, J.L.; Miller, R.A. Texaphyrins. Biochem. Pharmacol. 2000, 59, 733–739. [Google Scholar] [CrossRef]
- Tsolekile, N.; Nelana, S.; Oluwafemi, O.S. Porphyrin as diagnostic and therapeutic agent. Molecules 2019, 24, 2669. [Google Scholar] [CrossRef] [Green Version]
- Habermeyer, B.; Guilard, R. Some activities of PorphyChem illustrated by the applications of porphyrinoids in PDT, PIT and PDI. Photochem. Photobiol. Sci. 2018, 17, 1675–1690. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, R.; Yu, H.; Xu, Z.; Kang, Y.; Cui, H.; Xue, P. MnO2-capped silk fibroin (SF) nanoparticles with chlorin e6 (Ce6) encapsulation for augmented photo-driven therapy by modulating the tumor microenvironment. J. Mater. Chem. B 2021, 9, 3677–3688. [Google Scholar] [CrossRef]
- Sanhueza, C.; Hermosilla, J.; Bugallo-Casal, A.; Da Silva-Candal, A.; Taboada, C.; Millán, R.; Acevedo, F. One-step electrospun scaffold of dual-sized gelatin/poly-3-hydroxybutyrate nano/microfibers for skin regeneration in diabetic wound. Mater. Sci. Eng. C 2020, 119, 111602. [Google Scholar] [CrossRef]
- Synytsya, A.; Grafová, M.; Slepicka, P.; Gedeon, O.; Synytsya, A. Modification of chitosan–methylcellulose composite films with meso-tetrakis(4-sulfonatophenyl) porphyrin. Biomacromolecules 2011, 13, 489–498. [Google Scholar] [CrossRef]
- Zhao, L.; Qu, R.; Li, A.; Ma, R.; Shi, L. Cooperative self-assembly of porphyrins with polymers possessing bioactive functions. Chem. Commun. 2016, 52, 13543–13555. [Google Scholar] [CrossRef]
- Seema, A.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar]
- Ol’khov, A.A.; Tyubaeva, P.M.; Zernova, Y.N.; Kurnosov, A.S.; Karpova, S.G.; Iordanskii, A.L. Structure and properties of biopolymeric fibrous materials based on polyhydroxybutyrate–metalloporphyrin complexes. Russ. J. Gen. Chem. 2021, 91, 546–553. [Google Scholar] [CrossRef]
- Arai, T.; Tanaka, M.; Kawakami, H. Porphyrin-containing electrospun nanofibers: Positional control of porphyrin molecules in nanofibers and their catalytic application. ACS Appl. Mater. Interfaces 2012, 4, 5453–5457. [Google Scholar] [CrossRef]
- Gangemi, C.M.A.; Iudici, M.; Spitaleri, L.; Randazzo, R.; Gaeta, M.; D’Urso, A.; Fragalà, M.E. Polyethersulfone mats functionalized with porphyrin for removal of para-nitroaniline from aqueous solution. Molecules 2019, 24, 3344. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Song, L.; Zhang, X.; Zhou, R.; Yin, J.; Luan, S. Poly (γ-glutamic acid)-based electrospun nanofibrous mats with photodynamic therapy for effectively combating wound infection. Mater. Sci. Eng. C 2020, 113, 110936. [Google Scholar] [CrossRef]
- Kaerkitcha, N.; Sagawa, T. Amplified polarization properties of electrospun nanofibers containing fluorescent dyes and helical polymer. Photochem. Photobiol. Sci. 2018, 17, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Naznin, S.; Min, W. PHBV/PLLA-based composite scaffolds fabricated using an emulsion freezing/freeze-drying technique for bone tissue engineering: Surface modification and in vitro biological evaluation. Biofabrication 2012, 4, 015003. [Google Scholar]
- Nakayama, D.; Wu, F.; Mohanty, A.K.; Hirai, S.; Misra, M. Biodegradable composites developed from PBAT/PLA binary blends and silk powder: Compatibilization and performance evaluation. ACS Omega 2018, 3, 12412–12421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nofar, M.; Salehiyan, R.; Ciftci, U.; Jalali, A.; Durmuş, A. Ductility improvements of PLA-based binary and ternary blends with controlled morphology using PBAT, PBSA, and nanoclay. Compos. B Eng. 2019, 182, 107661. [Google Scholar] [CrossRef]
- Huang, M.-H.; Li, S.; Hutmacher, D.W.; Coudane, J.; Vert, M. Degradation characteristics of poly(ε-caprolactone)-based copolymers and blends. J. Appl. Polym. Sci. 2016, 102, 1681–1687. [Google Scholar] [CrossRef]
- Rajan, K.P.; Thomas, S.P.; Gopanna, A.; Chavali, M. Polyhydroxybutyrate (PHB): A Standout Biopolymer for Environmental Sustainability. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 1–23. [Google Scholar]
- Woolnough, C.A.; Yee, L.H.; Charlton, T.S.; Foster, L.J.R. Environmental degradation and biofouling of green plastics including short and medium chain length polyhydroxyalkanoates. Polym. Int. 2010, 59, 658–667. [Google Scholar] [CrossRef]
- Pati, S.; Maity, S.; Dash, A.; Jema, S.; Mohapatra, S.; Das, S.; Samantaray, D.P. Biocompatible PHB production from Bacillus species under submerged and solid-state fermentation and extraction through different downstream processing. Curr. Microbiol. 2020, 77, 1203–1209. [Google Scholar] [CrossRef]
- Sreedevi, S.; Unni, K.N.; Sajith, S.; Priji, P.; Josh, M.S.; Benjamin, S. Bioplastics: Advances in polyhydroxybutyrate research. In Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–30. [Google Scholar]
- Arrieta, M.P.; López, J.; Hernández, A.; Rayón, E. Ternary PLA–PHB–limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Kumara, B.P.; Maruthi, Y.P.; Veera Pratap, S.; Sudhakar, K.; Sadiku, R.; Prabhakar, M.N.; Il Song, J.; Subha, M.C.S.; Chowdoji Rao, K. Development and characterization of polycaprolactone (PCL)/poly ((R)-3-hydroxybutyric acid) (PHB) blend microspheres for tamoxifen drug relese studies. Int. J. Pharm. Pharm. Sci. 2015, 7, 95–100. [Google Scholar]
- Karimi, A.; Karbasi, S.; Razavi, S.; Zargar, E. Poly(hydroxybutyrate)/chitosan aligned electrospun scaffold as a novel substrate for nerve tissue engineering. Adv. Biomed. Eng. 2018, 7, 44. [Google Scholar]
- Saad, B.; Neuenschwander, P.; Uhlschmid, G.; Suter, U. New versatile, elastomeric, degradable polymeric materials for medicine. Int. J. Biol. Macromol. 1999, 25, 293–301. [Google Scholar] [CrossRef]
- Kim, G.M.; Wutzler, A.; Radusch, H.J.; Michler, G.H.; Simon, P.; Sperling, R.A.; Parak, W.J. One-dimension arrangement of gold nano-particles by electrospinning. Chem. Mater. 2005, 17, 4949–4957. [Google Scholar] [CrossRef]
- Dror, Y.; Salalha, W.; Khalfin, R.L.; Cohen, Y.; Yarin, A.L.; Zussman, E. Carbon nanotubes embeded in oriented polymer nanofibers by electrospinning. Langmuir 2003, 19, 7012–7020. [Google Scholar] [CrossRef]
- Jun, Z.; Aigner, A.; Czubayko, F.; Kissel, T.; Wendorff, J.H.; Greiner, A. Poly (vinyl alcohol) nanofibers by electrospinning as a protein delivery system and retardation of enzyme release by additional polymer coatings. Biomacromolecules 2005, 6, 1484–1488. [Google Scholar]
- Wang, H.S.; Fu, G.D.; Li, X.S. Functional polymeric nanofibers from electrospinning. Recent Pat. Nanotechnol. 2009, 3, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Joung, K.; Bae, J.W.; Park, K.D. Controlled release of heparin-binding growth factors using heparin-containing particulate systems for tissue regeneration. Expert Opin. Drug Deliv. 2008, 5, 1173–1184. [Google Scholar] [CrossRef]
- Lu, Y.; Berry, S.M.; Pfister, T.D. Engineering novel metalloproteins: Design of metal-binding sites into native protein scaffolds. Chem. Rev. 2001, 101, 3047–3080. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, C.; Li, B. Self-assembly of hemin on carbon nanotube as highly active peroxidase mimetic and its application for biosensing. RSC Adv. 2013, 3, 6044. [Google Scholar] [CrossRef]
- Qu, R.; Shen, L.; Chai, Z.; Jing, C.; Zhang, Y.; An, Y.; Shi, L. Hemin-block copolymer micelle as an artificial peroxidase and its applications in chromogenic detection and biocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 19207–19216. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Y.; Chen, G.; Li, G. Regulation of thrombin activity with a bifunctional aptamer and hemin: Development of a new anticoagulant and antidote pair. ChemBioChem 2009, 10, 2171–2176. [Google Scholar] [CrossRef]
- Alsharabasy, A.M.; Pandit, A.; Farràs, P. Recent advances in the design and sensing applications of hemin/coordination polymer-based nanocomposites. Adv. Mater. 2020, 33, 2003883. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Wei, W.; Li, Y.; Liu, A.; Zhang, Y.; Liu, S. Effect of annealing temperature and element composition of titanium dioxide/graphene/hemin catalysts for oxygen reduction reaction. RSC Adv. 2015, 5, 82879–82886. [Google Scholar] [CrossRef]
- Nitzan, Y.; Ladan, H.; Gozansky, S.; Malik, Z. Characterization of hemin antibacterial action on Staphylococcus aureus. FEMS Microbiol. Lett. 1987, 48, 401–406. [Google Scholar] [CrossRef]
- Dell’Acqua, S.; Massardi, E.; Monzani, E.; Di Natale, G.; Rizzarelli, E.; Casella, L. Interaction between hemin and prion peptides: Binding, oxidative reactivity and aggregation. Int. J. Mol. Sci. 2020, 21, 7553. [Google Scholar] [CrossRef]
- Zozulia, O.; Korendovych, I.V. Semi-rationally designed short peptides self-assemble and bind hemin to promote cyclopropanation. Angew. Chem. Int. Ed. 2020, 59, 8108–8112. [Google Scholar] [CrossRef]
- Sedaghat, S.; Shamspur, T.; Mohamadi, M.; Mostafavi, A. Extraction and preconcentration of hemin from human blood serum and breast cancer supernatant. J. Sep. Sci. 2015, 38, 4286–4291. [Google Scholar] [CrossRef]
- Dong, L.; Zang, J.; Wang, W.; Liu, X.; Zhang, Y.; Su, J.; Li, J. Electrospun single iron atoms dispersed carbon nanofibers as high performance electrocatalysts toward oxygen reduction reaction in acid and alkaline media. J. Colloid Interface Sci. 2019, 564, 134–142. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Serio, A.; Amdursky, N.; Besnard, C.; Stevens, M.M. Fabrication of hemin-doped serum albumin-based fibrous scaffolds for neural tissue engineering applications. ACS Appl. Mater. Interfaces 2018, 10, 5305–5317. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chou, W.; Liu, L.; Cui, Y.; Xue, P.; Jia, M. Electrochemical sensors fabricated by electrospinning technology: An overview. Sensors 2019, 19, 3676. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.D.; Longo, F.R.; Kampas, F.; Kim, J. On the preparation of metalloporphyrins. J. Radioanal. Nucl. Chem. 1970, 32, 2443–2445. [Google Scholar] [CrossRef]
- Lubasova, D.; Martinova, L. Controlled morphology of porous polyvinyl butyral nanofibers. J. Nanomater. 2011, 6, 1–6. [Google Scholar] [CrossRef]
- You, Y.; Youk, J.H.; Lee, S.W.; Min, B.M.; Lee, S.J.; Park, W.H. Preparation of porous ultrafine PGA fibers via selective dissolution of electrospun PGA/PLA blend fibers. Mater. Lett. 2006, 60, 757–760. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Koga, N.; Schick, C.V. Handbook of Thermal Analysis and Calorimetry: Applications to Polymers and Plastic; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Scandola, M.; Focarete, M.L.; Adamus, G.; Sikorska, W.; Baranowska, I.; Świerczek, S.; Jedliński, Z. Polymer blends of natural poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and a synthetic atactic poly (3-hydroxybutyrate). Characterization and biodegradation studies. Macromolecules 1997, 30, 2568–2574. [Google Scholar] [CrossRef]
- Liang, Z.; Freed, J.H. An assessment of the applicability of multifrequency ESR to study the complex dynamics of biomolecules. J. Phys. Chem. 1999, 103, 6384. [Google Scholar] [CrossRef]
- Sezer, D.; Freed, J.H.; Roux, B. Simulating electron spin resonance spectra of nitroxide spin labels from molecular dynamics and stochastic trajectories. J. Chem. Phys. 2008, 128, 165106-1–165106-16. [Google Scholar] [CrossRef] [Green Version]
- Katsogiannis, K.A.G.; Vladisavljević, G.T.; Georgiadou, S. Porous electrospun polycaprolactone (PCL) fibres by phase separation. Eur. Polym. J. 2015, 69, 284–295. [Google Scholar] [CrossRef] [Green Version]
- Domaschke, S.; Zündel, M.; Mazza, E.; Ehret, A.E. A 3D computational model of electrospun networks and its application to inform a reduced modelling approach. Int. J. Solids Struct. 2019, 178, 76–89. [Google Scholar] [CrossRef]
- Greenfeld, I.; Arinstein, A.; Fezzaa, K.; Rafailovich, M.H.; Zussman, E. Polymer dynamics in semidilute solution during electrospinning: A simple model and experimental observations. Phys. Rev. 2011, 84, 041806. [Google Scholar] [CrossRef] [Green Version]
- Mota, C.; Puppi, D.; Dinucci, D.; Gazzarri, M.; Chiellini, F. Additive manufacturing of star poly(ε-caprolactone) wet-spun scaffolds for bone tissue engineering applications. J. Bioact. Compat. Polym. 2013, 28, 320–337. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Davis, G.T.; Lauritzen, J.I. Treatise on Solid State Chemistry, Crystalline and Noncrystalline Solids, 3rd ed.; Plenum Press: New York, NY, USA, 1976; pp. 497–498. [Google Scholar]
- Reneker, D.H.; Yarin, A.L.; Fong, H.; Koombhongse, S. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J. Appl. Phys. 2000, 87, 4531–4547. [Google Scholar] [CrossRef] [Green Version]
- Karpova, S.G.; Olkhov, A.A.; Bakirov, A.V.; Chvalun, S.N.; Shilkina, N.S.; Popov, A.A. Poly (3-hydroxybutyrate) matrices modified with iron (III) complex with tetraphenylporphyrin. Analysis of structural and dynamic parameters. Chem. Phys. 2018, 37, 64–77. [Google Scholar]
- Sun, X.; Gao, N.; Li, Q.; Zhang, J.; Yang, X.; Ren, Z.; Yan, S. Crystal morphology of poly (3-hydroxybutyrate) on amorphous poly (vinylphenol) substrate. Langmuir 2016, 32, 3983–3994. [Google Scholar] [CrossRef]
- El-Hadi, A.M. Influence of electrospinning parameters on fiber diameter and mechanical properties of poly(3-Hydroxybutyrate) (PHB) and polyanilines (PANI) blends. Polymers 2016, 8, 97. [Google Scholar] [CrossRef] [Green Version]
- Barham, P.J.; Keller, A.; Otun, E.L.; Holmes, P.A. Crystallization and morphology of a bacterial thermoplastic: Poly-3-hydroxybutyrate. J. Mater. Sci. 1984, 19, 2781–2794. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarian, A.L.; Zussman, E.; Xu, H. Electrospinning of nanofibers from polymer solutions and melts. Adv. Appl. Mech. 2007, 41, 43–195. [Google Scholar]
- Baumgarten, P. Electrostatic spinning of acrylic microfibers. J. Colloid Interface Sci. 1971, 36, 71–79. [Google Scholar] [CrossRef]
- Syerko, E.; Comas-Cardona, S.; Binetruy, C. Models of mechanical properties/behavior of dry fibrous materials at various scales in bending and tension: A review. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1365–1388. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent advances in the development of biodegradable PHB-based toughening materials: Approaches, advantages and applications. Mater. Sci. Eng. C 2018, 92, 1092–1116. [Google Scholar] [CrossRef]
- Chan, C.H.; Kummerlöwe, C.; Kammer, H.W. Crystallization and melting behavior of poly(3-hydroxybutyrate)-based blends. Macromol. Chem. Phys. 2004, 205, 664–675. [Google Scholar] [CrossRef]
- Haslböck, M.; Klotz, M.; Steiner, L.; Sperl, J.; Sieber, V.; Zollfrank, C.; Van Opdenbosch, D. Structures of mixed-tacticity polyhydroxybutyrates. Macromolecules 2018, 51, 5001–5010. [Google Scholar] [CrossRef]











| Sample | Density, g/cm3 (±S.D., n = 10) | Average Diameter, µm (±S.D., n = 100) | Pore Size, µm (±S.D., n = 50) | Porosity, % (±S.D., n = 50) |
|---|---|---|---|---|
| PHB 0 wt. % | 0.30 ± 0.01 | 3.50 ± 0.08 | 15 ± 10 | 80 ± 2.0 |
| PHB with 1 wt. % of hemin | 0.20 ± 0.02 | 2.06 ± 0.07 | 109 ± 10 | 92 ± 1.5 |
| PHB with 3 wt. % of hemin | 0.20 ± 0.01 | 1.77 ± 0.04 | 83 ± 10 | 92 ± 1.5 |
| PHB mats with 5 wt. % of hemin | 0.17 ± 0.01 | 1.77 ± 0.04 | 52 ± 10 | 94 ± 1.2 |
| Sample | Tensile Strength, Mpa Δ ± 0.02 MPa | Elongation at Break, % Δ ± 0.2% |
|---|---|---|
| PHB with 0 wt. % | 1.7 | 3.6 |
| PHB with 1 wt. % of hemin | 0.7 | 4.7 |
| PHB with 3 wt. % of hemin | 1.9 | 4.7 |
| PHB with 5 wt. % of hemin | 5.5 | 6.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyubaeva, P.; Varyan, I.; Lobanov, A.; Olkhov, A.; Popov, A. Effect of the Hemin Molecular Complexes on the Structure and Properties of the Composite Electrospun Materials Based on Poly(3-hydroxybutyrate). Polymers 2021, 13, 4024. https://doi.org/10.3390/polym13224024
Tyubaeva P, Varyan I, Lobanov A, Olkhov A, Popov A. Effect of the Hemin Molecular Complexes on the Structure and Properties of the Composite Electrospun Materials Based on Poly(3-hydroxybutyrate). Polymers. 2021; 13(22):4024. https://doi.org/10.3390/polym13224024
Chicago/Turabian StyleTyubaeva, Polina, Ivetta Varyan, Anton Lobanov, Anatoly Olkhov, and Anatoly Popov. 2021. "Effect of the Hemin Molecular Complexes on the Structure and Properties of the Composite Electrospun Materials Based on Poly(3-hydroxybutyrate)" Polymers 13, no. 22: 4024. https://doi.org/10.3390/polym13224024
APA StyleTyubaeva, P., Varyan, I., Lobanov, A., Olkhov, A., & Popov, A. (2021). Effect of the Hemin Molecular Complexes on the Structure and Properties of the Composite Electrospun Materials Based on Poly(3-hydroxybutyrate). Polymers, 13(22), 4024. https://doi.org/10.3390/polym13224024