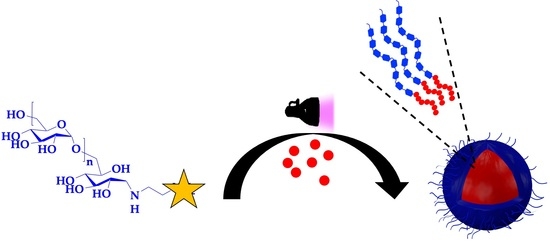
Dextran-Coated Latex Nanoparticles via Photo-RAFT Mediated Polymerization Induced Self-Assembly
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Instruments
2.2.1. NMR Spectroscopy
2.2.2. Size Exclusion Chromatography (SEC)
2.2.3. Dynamic/Static Light Scattering (DLS/SLS)
2.2.4. Transmission Electron Microscopy (TEM)
2.3. Synthesis
2.3.1. Preparation of Macromolecular Chain Transfer Agent (eDexCTA)
2.3.2. Latex Formulation via Photo-RAFT PISA
3. Results and Discussion
3.1. Synthesis of the Macromolecular Chain Transfer Agent (eDexCTA)
3.2. Latex Formulation via Photo-RAFT PISA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pichot, C. Surface-functionalized latexes for biotechnological applications. Curr. Opin. Colloid Interface Sci. 2004, 9, 213–221. [Google Scholar] [CrossRef]
- Guyot, A.; Landfester, K.; Joseph Schork, F.; Wang, C. Hybrid polymer latexes. Prog. Polym. Sci. 2007, 32, 1439–1461. [Google Scholar] [CrossRef]
- Fuentes-Exposito, M.; Norsic, S.; Février, T.; Dugas, P.-Y.; Boutti, S.; Devisme, S.; Bonnet, A.; D’Agosto, F.; Lansalot, M. Surfactant-free emulsion polymerization of vinylidene fluoride mediated by RAFT/MADIX reactive poly(ethylene glycol) polymer chains. Polym. Chem. 2021, 12, 5640–5649. [Google Scholar] [CrossRef]
- Smeets, N.M.B.; Imbrogno, S.; Bloembergen, S. Carbohydrate functionalized hybrid latex particles. Carbohydr. Polym. 2017, 173, 233–252. [Google Scholar] [CrossRef]
- Jiménez Saelices, C.; Save, M.; Capron, I. Synthesis of latex stabilized by unmodified cellulose nanocrystals: The effect of monomers on particle size. Polym. Chem. 2019, 10, 727–737. [Google Scholar] [CrossRef]
- Boujemaoui, A.; Cobo Sanchez, C.; Engström, J.; Bruce, C.; Fogelström, L.; Carlmark, A.; Malmström, E. Polycaprolactone Nanocomposites Reinforced with Cellulose Nanocrystals Surface-Modified via Covalent Grafting or Physisorption: A Comparative Study. ACS Appl. Mater. Interfaces 2017, 9, 35305–35318. [Google Scholar] [CrossRef]
- Yan, X.; Chai, L.; Fleury, E.; Ganachaud, F.; Bernard, J. ‘Sweet as a Nut’: Production and use of nanocapsules made of glycopolymer or polysaccharide shell. Prog. Polym. Sci. 2021, 120, 101429. [Google Scholar] [CrossRef]
- Su, L.; Feng, Y.; Wei, K.; Xu, X.; Liu, R.; Chen, G. Carbohydrate-Based Macromolecular Biomaterials. Chem. Rev. 2021, 121, 10950–11029. [Google Scholar] [CrossRef] [PubMed]
- Ferji, K.; Nouvel, C.; Babin, J.; Albouy, P.-A.; Li, M.-H.; Six, J.-L. Controlled synthesis of new amphiphilic glycopolymers with liquid crystal grafts. J. Polym. Sci. A Polym. Chem. 2013, 51, 3829–3839. [Google Scholar] [CrossRef]
- Xu, J.; Hu, H. Preparation and characterization of styrene acrylate emulsion surface sizing agent modified with rosin. J. Appl. Polym. Sci. 2012, 123, 611–616. [Google Scholar] [CrossRef]
- Rotureau, E.; Raynaud, J.; Choquenet, B.; Marie, E.; Nouvel, C.; Six, J.L.; Dellacherie, E.; Durand, A. Application of amphiphilic polysaccharides as stabilizers in direct and inverse free-radical miniemulsion polymerization. Colloids Surf. A Physicochem. Eng. Asp. 2008, 331, 84–90. [Google Scholar] [CrossRef]
- Wu, M.; Dellacherie, E.; Durand, A.; Marie, E. Poly(n-butyl cyanoacrylate) nanoparticles via miniemulsion polymerization (1): Dextran-based surfactants. Colloids Surf. B 2009, 69, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Rotureau, E.; Marie, E.; Dellacherie, E.; Durand, A. From polymeric surfactants to colloidal systems (3): Neutral and anionic polymeric surfactants derived from dextran. Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 229–238. [Google Scholar] [CrossRef]
- Roy, A.; Murcia Valderrama, M.A.; Daujat, V.; Ferji, K.; Léonard, M.; Durand, A.; Babin, J.; Six, J.-L. Stability of a biodegradable microcarrier surface: Physically adsorbed versus chemically linked shells. J. Mater. Chem. B 2018, 6, 5130–5143. [Google Scholar] [CrossRef]
- El Founi, M.; Laroui, H.; Canup, B.S.B.; Ametepe, J.S.; Vanderesse, R.; Acherar, S.; Babin, J.; Ferji, K.; Chevalot, I.; Six, J.-L. Doxorubicin Intracellular Release Via External UV Irradiation of Dextran-g-poly(o-nitrobenzyl acrylate) Photosensitive Nanoparticles. ACS Appl. Bio Mater. 2021, 4, 2742–2751. [Google Scholar] [CrossRef]
- Laville, M.; Babin, J.; Londono, I.; Legros, M.; Nouvel, C.; Durand, A.; Vanderesse, R.; Leonard, M.; Six, J.-L. Polysaccharide-covered nanoparticles with improved shell stability using click-chemistry strategies. Carbohydr. Polym. 2013, 93, 537–546. [Google Scholar] [CrossRef]
- Nouvel, C.; Frochot, C.; Sadtler, V.; Dubois, P.; Dellacherie, E.; Six, J.-L. Polylactide-Grafted Dextrans: Synthesis and Properties at Interfaces and in Solution. Macromolecules 2004, 37, 4981–4988. [Google Scholar] [CrossRef]
- Schatz, C.; Louguet, S.; Meins, J.F.L.; Lecommandoux, S. Polysaccharide-block-polypeptide copolymer vesicles: Towards synthetic viral capsids. Angew. Chem. Int. Ed. 2009, 48, 2572–2575. [Google Scholar] [CrossRef]
- Ferji, K.; Nouvel, C.; Babin, J.; Li, M.-H.; Gaillard, C.; Nicol, E.; Chassenieux, C.; Six, J.-L. Polymersomes from Amphiphilic Glycopolymers Containing Polymeric Liquid Crystal Grafts. ACS Macro Lett. 2015, 4, 1119–1122. [Google Scholar] [CrossRef]
- Peyret, A.; Trant, J.F.; Bonduelle, C.V.; Ferji, K.; Jain, N.; Lecommandoux, S.; Gillies, E.R. Synthetic glycopolypeptides: Synthesis and self-assembly of poly(gamma-benzyl-L-glutamate)-glycosylated dendron hybrids. Polym. Chem. 2015, 6, 7902–7912. [Google Scholar] [CrossRef]
- Ikkene, D.; Arteni, A.A.; Ouldali, M.; Six, J.-L.; Ferji, K. Self-assembly of amphiphilic copolymers containing polysaccharide: PISA versus nanoprecipitation, and the temperature effect. Polym. Chem. 2020, 11, 4729–4740. [Google Scholar] [CrossRef]
- Ikkene, D.; Arteni, A.A.; Song, H.; Laroui, H.; Six, J.L.; Ferji, K. Synthesis of dextran-based chain transfer agent for RAFT-mediated polymerization and glyco-nanoobjects formulation. Carbohydr. Polym. 2020, 234, 115943. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Armes, S.P. RAFT Synthesis of Sterically Stabilized Methacrylic Nanolatexes and Vesicles by Aqueous Dispersion Polymerization. Angew. Chem. Int. Ed. 2010, 49, 4042–4046. [Google Scholar] [CrossRef] [PubMed]
- Delaittre, G.; Nicolas, J.; Lefay, C.; Save, M.; Charleux, B. Surfactant-free synthesis of amphiphilic diblock copolymer nanoparticles via nitroxide-mediated emulsion polymerization. Chem. Commun. 2005, 614–616. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.-M.; Sun, X.-L.; Pan, C.-Y. Morphology Transition in RAFT Polymerization for Formation of Vesicular Morphologies in One Pot. Macromolecules 2009, 42, 4950–4952. [Google Scholar] [CrossRef]
- Deane, O.J.; Jennings, J.; Armes, S.P. Shape-shifting thermoreversible diblock copolymer nano-objects via RAFT aqueous dispersion polymerization of 4-hydroxybutyl acrylate. Chem. Sci. 2021, 12, 13719–13729. [Google Scholar] [CrossRef]
- Cao, J.; Tan, Y.; Chen, Y.; Zhang, L.; Tan, J. Expanding the Scope of Polymerization-Induced Self-Assembly: Recent Advances and New Horizons. Macromol. Rapid Commun. 2021, 2100498. [Google Scholar] [CrossRef]
- Xu, S.; Corrigan, N.; Boyer, C. Forced gradient copolymerisation: A simplified approach for polymerisation-induced self-assembly. Polym. Chem. 2021, 12, 57–68. [Google Scholar] [CrossRef]
- Phan, H.; Taresco, V.; Penelle, J.; Couturaud, B. Polymerisation-induced self-assembly (PISA) as a straightforward formulation strategy for stimuli-responsive drug delivery systems and biomaterials: Recent advances. Biomater. Sci. 2021, 9, 38–50. [Google Scholar] [CrossRef]
- Shahrokhinia, A.; Scanga, R.A.; Biswas, P.; Reuther, J.F. PhotoATRP-Induced Self-Assembly (PhotoATR-PISA) Enables Simplified Synthesis of Responsive Polymer Nanoparticles in One-Pot. Macromolecules 2021, 54, 1441–1451. [Google Scholar] [CrossRef]
- Grazon, C.; Salas-Ambrosio, P.; Ibarboure, E.; Buol, A.; Garanger, E.; Grinstaff, M.W.; Lecommandoux, S.; Bonduelle, C. Aqueous Ring-Opening Polymerization-Induced Self-Assembly (ROPISA) of N-Carboxyanhydrides. Angew. Chem. Int. Ed. 2020, 59, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Ferji, K.; Venturini, P.; Cleymand, F.; Chassenieux, C.; Six, J.-L. In situ glyco-nanostructure formulation via photo-polymerization induced self-assembly. Polym. Chem. 2018, 9, 2868–2872. [Google Scholar] [CrossRef]
- Six, J.L.; Ferji, K. Polymerization induced self-assembly: An opportunity toward the self-assembly of polysaccharide-containing copolymers into high-order morphologies. Polym. Chem. 2019, 10, 45–53. [Google Scholar] [CrossRef]
- Ikkene, D.; Arteni, A.A.; Ouldali, M.; Francius, G.; Brûlet, A.; Six, J.-L.; Ferji, K. Direct Access to Polysaccharide-Based Vesicles with a Tunable Membrane Thickness in a Large Concentration Window via Polymerization-Induced Self-Assembly. Biomacromolecules 2021, 22, 3128–3137. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.; Save, M.; Arathoon, B.; Charleux, B. Preparation of a xanthate-terminated dextran by click chemistry: Application to the synthesis of polysaccharide-coated nanoparticles via surfactant-free ab initio emulsion polymerization of vinyl acetate. J. Polym. Sci. A Polym. Chem. 2008, 46, 2845–2857. [Google Scholar] [CrossRef]
- Kapishon, V.; Whitney, R.A.; Champagne, P.; Cunningham, M.F.; Neufeld, R.J. Polymerization Induced Self-Assembly of Alginate Based Amphiphilic Graft Copolymers Synthesized by Single Electron Transfer Living Radical Polymerization. Biomacromolecules 2015, 16, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Hatton, F.L.; Ruda, M.; Lansalot, M.; D’Agosto, F.; Malmström, E.; Carlmark, A. Xyloglucan-Functional Latex Particles via RAFT-Mediated Emulsion Polymerization for the Biomimetic Modification of Cellulose. Biomacromolecules 2016, 17, 1414–1424. [Google Scholar] [CrossRef]
- Chaduc, I.; Reynaud, E.; Dumas, L.; Albertin, L.; D’Agosto, F.; Lansalot, M. From well-defined poly(N-acryloylmorpholine)-stabilized nanospheres to uniform mannuronan- and guluronan-decorated nanoparticles by RAFT polymerization-induced self-assembly. Polymer 2016, 106, 218–228. [Google Scholar] [CrossRef]
- Ting, S.R.S.; Min, E.H.; Zetterlund, P.B.; Stenzel, M.H. Controlled/Living ab Initio Emulsion Polymerization via a Glucose RAFTstab: Degradable Cross-Linked Glyco-Particles for Concanavalin A/FimH Conjugations to Cluster E. coli Bacteria. Macromolecules 2010, 43, 5211–5221. [Google Scholar] [CrossRef]
- Ladmiral, V.; Semsarilar, M.; Canton, I.; Armes, S.P. Polymerization-Induced Self-Assembly of Galactose-Functionalized Biocompatible Diblock Copolymers for Intracellular Delivery. J. Am. Chem. Soc. 2013, 135, 13574–13581. [Google Scholar] [CrossRef]
- Chen, S.; Alves, M.-H.; Save, M.; Billon, L. Synthesis of amphiphilic diblock copolymers derived from renewable dextran by nitroxide mediated polymerization: Towards hierarchically structured honeycomb porous films. Polym. Chem. 2014, 5, 5310–5319. [Google Scholar] [CrossRef]
- Houga, C.; Meins, J.F.L.; Borsali, R.; Taton, D.; Gnanou, Y. Synthesis of ATRP-induced dextran-b-polystyrene diblock copolymers and preliminary investigation of their self-assembly in water. Chem. Commun. 2007, 3063–3065. [Google Scholar] [CrossRef] [PubMed]
- Mane, S.R. Trending methods employed for polymerization induced self-assembly. New J. Chem. 2020, 44, 6690–6698. [Google Scholar] [CrossRef]
- Li, S.; Han, G.; Zhang, W. Cross-linking approaches for block copolymer nano-assemblies via RAFT-mediated polymerization-induced self-assembly. Polym. Chem. 2020, 11, 4681–4692. [Google Scholar] [CrossRef]
- D’Agosto, F.; Rieger, J.; Lansalot, M. RAFT-Mediated Polymerization-Induced Self-Assembly. Angew. Chem. Int. Ed. 2020, 59, 8368–8392. [Google Scholar] [CrossRef] [PubMed]
- Lertturongchai, P.; Ibrahim, M.I.A.; Durand, A.; Sunintaboon, P.; Ferji, K. Synthesis of Thermoresponsive Copolymers with Tunable UCST-Type Phase Transition Using Aqueous Photo-RAFT Polymerization. Macromol. Rapid Commun. 2020, 41, 2000058. [Google Scholar] [CrossRef]
- Nomeir, B.; Fabre, O.; Ferji, K. Effect of Tertiary Amines on the Photoinduced Electron Transfer-Reversible Addition–Fragmentation Chain Transfer (PET-RAFT) Polymerization. Macromolecules 2019, 52, 6898–6903. [Google Scholar] [CrossRef]
- Fonseca Parra, E.P.; Chouchene, B.; Six, J.-L.; Schneider, R.; Ferji, K. Mechanistic Insights into Oxygen Tolerance of Graphitic Carbon Nitride-Mediated Heterogeneous Photoinduced Electron Transfer-Reversible Addition Fragmentation Chain Transfer Polymerization. ACS Appl. Polym. Mater. 2021, 3, 3649–3658. [Google Scholar] [CrossRef]
- Khalil, A.; Gerardin-Charbonnier, C.; Chapuis, H.; Ferji, K.; Six, J.-L. Original Bio-Based Antioxidant Poly(meth)acrylate from Gallic Acid-Based Monomers. ACS Sustain. Chem. Eng. 2021, 9, 11458–11468. [Google Scholar] [CrossRef]
- Semsarilar, M.; Abetz, V. Polymerizations by RAFT: Developments of the Technique and Its Application in the Synthesis of Tailored (Co)polymers. Macromol. Chem. Phys. 2021, 222, 2000311. [Google Scholar] [CrossRef]
- Galinsky, G.; Burchard, W. Starch Fractions as Examples for Nonrandomly Branched Macromolecules. 3. Angular Dependence in Static Light Scattering. Macromolecules 1997, 30, 4445–4453. [Google Scholar] [CrossRef]
- Czajka, A.; Armes, S.P. In situ SAXS studies of a prototypical RAFT aqueous dispersion polymerization formulation: Monitoring the evolution in copolymer morphology during polymerization-induced self-assembly. Chem. Sci. 2020, 11, 11443–11454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zeng, R.; Zhang, Y.; Chen, Y.; Zhang, L.; Tan, J. Two Polymersome Evolution Pathways in One Polymerization-Induced Self-Assembly (PISA) System. Macromolecules 2020, 53, 8982–8991. [Google Scholar] [CrossRef]
- Zaquen, N.; Yeow, J.; Junkers, T.; Boyer, C.; Zetterlund, P.B. Visible Light-Mediated Polymerization-Induced Self-Assembly Using Continuous Flow Reactors. Macromolecules 2018, 51, 5165–5172. [Google Scholar] [CrossRef]
- Touve, M.A.; Wright, D.B.; Mu, C.; Sun, H.; Park, C.; Gianneschi, N.C. Block Copolymer Amphiphile Phase Diagrams by High-Throughput Transmission Electron Microscopy. Macromolecules 2019, 52, 5529–5537. [Google Scholar] [CrossRef]
- Varlas, S.; Georgiou, P.G.; Bilalis, P.; Jones, J.R.; Hadjichristidis, N.; O’Reilly, R.K. Poly(sarcosine)-Based Nano-Objects with Multi-Protease Resistance by Aqueous Photoinitiated Polymerization-Induced Self-Assembly (Photo-PISA). Biomacromolecules 2018, 19, 4453–4462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luppi, L.; Babut, T.; Petit, E.; Rolland, M.; Quemener, D.; Soussan, L.; Moradi, M.A.; Semsarilar, M. Antimicrobial polylysine decorated nano-structures prepared through polymerization induced self-assembly (PISA). Polym. Chem. 2019, 10, 336–344. [Google Scholar] [CrossRef]
- Fan, B.; Liu, Y.; Wan, J.; Crawford, S.; Thang, S.H. Polymerization-Induced Self-Assembly (PISA) and “Host–Guest” Complexation-Directed Polymer/Gold Nanocomposites. ACS Mater. Lett. 2020, 2, 492–498. [Google Scholar] [CrossRef]





| Suspension (SCDHX) | SC | X | Conv.(%) a | RTEM (nm) b | RH (nm) c | PDI d | Rg (nm) e | Rg/RH | Rfit(nm) |
|---|---|---|---|---|---|---|---|---|---|
| 5%DH50 | 5% | 50 | 90 | 28 ± 14 | 38 | 0.20 | - | - | - |
| 5%DH100 | 5% | 100 | >99 | 31 ± 4 | 33 | 0.19 | - | - | - |
| 5%DH200 | 5% | 200 | 98 | 37 ± 5 | 36 | 0.12 | - | - | - |
| 5%DH300 | 5% | 300 | 98 | 80 ± 24 | 64 | 0.07 | 46 | 0.72 | 60 |
| 7.5%DH50 | 7.5% | 50 | >99 | 17 ± 2 | 18 | 0.50 | - | - | - |
| 7.5%DH100 | 7.5% | 100 | 98 | 29 ± 4 | 31 | 0.24 | - | - | - |
| 7.5%DH200 | 7.5% | 200 | >99 | 40 ± 7 | 43 | 0.08 | 37 | 0.86 | 49 |
| 7.5%DH300 | 7.5% | 300 | 94 | 51 ± 6 | 54 | 0.01 | 42 | 0.77 | 55 |
| 10%DH50 | 10% | 50 | >99 | 16 ± 2 | 19 | 0.48 | - | - | - |
| 10%DH100 | 10% | 100 | >99 | 30 ± 4 | 32 | 0.24 | - | - | - |
| 10%DH200 | 10% | 200 | >99 | 39 ± 6 | 47 | 0.11 | 36 | 0.77 | 48 |
| 10%DH300 | 10% | 300 | 97 | 107 ± 18 | 99 | 0.01 | 75 | 0.76 | 95 |
| 12.5%DH50 | 12.5% | 50 | >99 | 21 ± 3 | 22 | 0.22 | - | - | - |
| 12.5%DH100 | 12.5% | 100 | >99 | 34 ± 6 | 32 | 0.08 | - | - | - |
| 12.5%DH200 | 12.5% | 200 | >99 | 59 ± 10 | 76 | 0.10 | 44 | 0.58 | 60 |
| 12.5%DH300 | 12.5% | 300 | 97 | 238 ± 72 | 175 | 0.05 | 134 | 0.77 | 175 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero Castro, V.L.; Nomeir, B.; Arteni, A.A.; Ouldali, M.; Six, J.-L.; Ferji, K. Dextran-Coated Latex Nanoparticles via Photo-RAFT Mediated Polymerization Induced Self-Assembly. Polymers 2021, 13, 4064. https://doi.org/10.3390/polym13234064
Romero Castro VL, Nomeir B, Arteni AA, Ouldali M, Six J-L, Ferji K. Dextran-Coated Latex Nanoparticles via Photo-RAFT Mediated Polymerization Induced Self-Assembly. Polymers. 2021; 13(23):4064. https://doi.org/10.3390/polym13234064
Chicago/Turabian StyleRomero Castro, Valeria Lizeth, Brahim Nomeir, Ana Andreea Arteni, Malika Ouldali, Jean-Luc Six, and Khalid Ferji. 2021. "Dextran-Coated Latex Nanoparticles via Photo-RAFT Mediated Polymerization Induced Self-Assembly" Polymers 13, no. 23: 4064. https://doi.org/10.3390/polym13234064
APA StyleRomero Castro, V. L., Nomeir, B., Arteni, A. A., Ouldali, M., Six, J. -L., & Ferji, K. (2021). Dextran-Coated Latex Nanoparticles via Photo-RAFT Mediated Polymerization Induced Self-Assembly. Polymers, 13(23), 4064. https://doi.org/10.3390/polym13234064