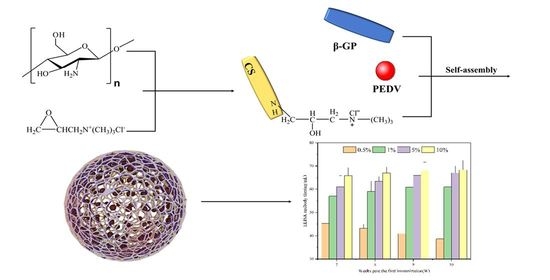
Self-Assembly of Soluble Chitosan Derivatives Nanoparticles for Vaccine: Synthesis, Characterization and Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the N-2-HACC
2.3. Characteristics of the N-2-HACC
2.3.1. FTIR and 1H NMR Spectroscopy
2.3.2. X-ray Diffraction
2.3.3. Degree of Deacetylation of CS
2.3.4. Viscosity Measurement
2.3.5. Degree of Substitution of N-2-HACC
2.3.6. Zeta Potential and Size Distribution
2.3.7. Solubility Test
2.4. Nanoparticle Synthesis and Immune Effect Evaluation
3. Results and Discussion
3.1. Characterization of the N-2-HACC
3.1.1. FTIR and 1H NMR Spectroscopy
3.1.2. XRD
3.1.3. Degree of Deacetylation of CS
3.1.4. Viscosity Measurement
3.1.5. Degree of Substitution of N-2-HACC
3.1.6. Zeta Potential
3.1.7. Solubility Test
3.2. Preparation of N-2-HACC NPs
3.3. N-2-HACC/PEDV Immune Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, K.; Saif, L.J.; Wang, Q. Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020, 286, 198045. [Google Scholar] [CrossRef]
- Jung, K.; Saif, L.J. Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015, 204, 134–143. [Google Scholar] [CrossRef]
- Song, D.; Moon, H.; Kang, B. Porcine epidemic diarrhea: A review of current epidemiology and available vaccines. Clin. Exp. Vaccine Res. 2015, 4, 166–176. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Zhu, H.; Xia, X.; Liang, Z.; Ma, X.; Sun, B. Vaccine adjuvants: Understanding the structure and mechanism of adjuvanticity. Vaccine 2019, 37, 3167–3178. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Gao, S.; Cui, X.; Sun, D.; Zhao, K. Adjuvants and delivery systems based on polymeric nanoparticles for mucosal vaccines. Int. J. Pharm. 2019, 572, 118731. [Google Scholar] [CrossRef] [PubMed]
- Moine, L.; Canali, M.M.; Porporatto, C.; Correa, S.G. Reviewing the biological activity of chitosan in the mucosa: Focus on intestinal immunity. Int. J. Biol. Macromol. 2021, 189, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, M.; Fényes, E.; Bartos, C.; Zeeshan, M.; Ambrus, R. Chitosan biopolymer, its derivatives and potential applications in nano-therapeutics: A comprehensive review. Eur. Polym. J. 2021, 160, 110767. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Liang, Y.; Deng, L.; Chen, C.; Zhang, J.; Zhou, R.; Li, X.; Hu, R.; Dong, A. Preparation and properties of thermoreversible hydrogels based on methoxy poly(ethylene glycol)-grafted chitosan nanoparticles for drug delivery systems. Carbohydr. Polym. 2011, 83, 1828–1833. [Google Scholar] [CrossRef]
- Shelma, R.; Sharma, C.P. Submicroparticles composed of amphiphilic chitosan derivative for oral insulin and curcumin release applications. Colloids Surf. B Biointerfaces 2011, 88, 722–728. [Google Scholar] [CrossRef]
- Yan, D.; Hu, S.; Zhou, Z.; Zeenat, S.; Cheng, F.; Li, Y.; Feng, C.; Cheng, X.; Chen, X. Different chemical groups modification on the surface of chitosan nonwoven dressing and the hemostatic properties. Int. J. Biol. Macromol. 2018, 107, 463–469. [Google Scholar] [CrossRef]
- Andreica, B.-I.; Cheng, X.; Marin, L. Quaternary ammonium salts of chitosan. A critical overview on the synthesis and properties generated by quaternization. Eur. Polym. J. 2020, 139, 110016. [Google Scholar] [CrossRef]
- Chakka, V.P.; Zhou, T. Carboxymethylation of polysaccharides: Synthesis and bioactivities. Int. J. Biol. Macromol. 2020, 165, 2425–2431. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Nwe, N.; Tokura, S.; Tamura, H. Sulfated chitin and chitosan as novel biomaterials. Int. J. Biol. Macromol. 2007, 40, 175–181. [Google Scholar] [CrossRef]
- Kazemi, M.S.; Mohammadi, Z.; Amini, M.; Yousefi, M.; Tarighi, P.; Eftekhari, S.; Rafiee Tehrani, M. Thiolated chitosan-lauric acid as a new chitosan derivative: Synthesis, characterization and cytotoxicity. Int. J. Biol. Macromol. 2019, 136, 823–830. [Google Scholar] [CrossRef]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Liu, W.-S.; Sun, J.-F.; Hou, G.-G.; Chen, Q.; Cong, W.; Zhao, F. Non-toxic O-quaternized chitosan materials with better water solubility and antimicrobial function. Int. J. Biol. Macromol. 2016, 84, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Dashtimoghadam, E.; Mirzadeh, H.; Taromi, F.A.; Nyström, B. Microfluidic self-assembly of polymeric nanoparticles with tunable compactness for controlled drug delivery. Polymer 2013, 54, 4972–4979. [Google Scholar] [CrossRef]
- Koukaras, E.N.; Papadimitriou, S.A.; Bikiaris, D.N.; Froudakis, G.E. Insight on the Formation of Chitosan Nanoparticles through Ionotropic Gelation with Tripolyphosphate. Mol. Pharm. 2012, 9, 2856–2862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tan, W.; Wang, G.; Yin, X.; Li, Q.; Dong, F.; Guo, Z. Synthesis, characterization, and the antioxidant activity of N,N,N-trimethyl chitosan salts. Int. J. Biol. Macromol. 2018, 118, 9–14. [Google Scholar] [CrossRef]
- Tang, F.; Lv, L.; Lu, F.; Rong, B.; Li, Z.; Lu, B.; Yu, K.; Liu, J.; Dai, F.; Wu, D.; et al. Preparation and characterization of N-chitosan as a wound healing accelerator. Int. J. Biol. Macromol. 2016, 93, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.D.; Vanjari, Y.H.; Sancheti, K.H.; Patel, H.M.; Belgamwar, V.S.; Surana, S.J.; Pardeshi, C.V. New nasal nanocomplex self-assembled from charged biomacromolecules: N,N,N-Trimethyl chitosan and dextran sulfate. Int. J. Biol. Macromol. 2016, 88, 476–490. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, T.; Bangde, P.; Dandekar, P.; Jain, R. Greener approach for synthesis of N,N,N-trimethyl chitosan (TMC) using ternary deep eutectic solvents (TDESs). Carbohydr. Res. 2020, 493, 108033. [Google Scholar] [CrossRef] [PubMed]
- Leceta, I.; Guerrero, P.; Ibarburu, I.; Dueñas, M.T.; de la Caba, K. Characterization and antimicrobial analysis of chitosan-based films. J. Food Eng. 2013, 116, 889–899. [Google Scholar] [CrossRef]
- He, G.; Ke, W.; Chen, X.; Kong, Y.; Zheng, H.; Yin, Y.; Cai, W. Preparation and properties of quaternary ammonium chitosan-g-poly(acrylic acid-co-acrylamide) superabsorbent hydrogels. React. Funct. Polym. 2017, 111, 14–21. [Google Scholar] [CrossRef]
- Bashir, S.; Teo, Y.Y.; Ramesh, S.; Ramesh, K. Synthesis, characterization, properties of N-succinyl chitosan-g-poly (methacrylic acid) hydrogels and in vitro release of theophylline. Polymer 2016, 92, 36–49. [Google Scholar] [CrossRef]
- Wu, M.; Long, Z.; Xiao, H.; Dong, C. Preparation of N, N, N-trimethyl chitosan via a novel approach using dimethyl carbonate. Carbohydr. Polym. 2017, 169, 83–91. [Google Scholar] [CrossRef]
- Abueva, C.; Ryu, H.S.; Min, J.W.; Chung, P.S.; You, H.S.; Yang, M.S.; Woo, S.H. Quaternary ammonium N,N,N-trimethyl chitosan derivative and povidone iodine complex as a potent antiseptic with enhanced wound healing property. Int. J. Biol. Macromol. 2021, 182, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, C.V.; Belgamwar, V.S. Controlled synthesis of N,N,N-trimethyl chitosan for modulated bioadhesion and nasal membrane permeability. Int. J. Biol. Macromol. 2016, 82, 933–944. [Google Scholar] [CrossRef]
- Wang, A.; Zhu, Q.; Xing, Z. Multifunctional quaternized chitosan@surface plasmon resonance Ag/N-TiO2 core-shell microsphere for synergistic adsorption-photothermal catalysis degradation of low-temperature wastewater and bacteriostasis under visible light. Chem. Eng. J. 2020, 393, 124781. [Google Scholar] [CrossRef]
- Wu, J.; Su, Z.-G.; Ma, G.-H. A thermo- and pH-sensitive hydrogel composed of quaternized chitosan/glycerophosphate. Int. J. Pharm. 2006, 315, 1–11. [Google Scholar] [CrossRef]
- Phuangkaew, T.; Booranabunyat, N.; Kiatkamjornwong, S.; Thanyasrisung, P.; Hoven, V.P. Amphiphilic quaternized chitosan: Synthesis, characterization, and anti-cariogenic biofilm property. Carbohydr Polym 2021, 118882. [Google Scholar] [CrossRef]
- Vallapa, N.; Wiarachai, O.; Thongchul, N.; Pan, J.; Tangpasuthadol, V.; Kiatkamjornwong, S.; Hoven, V.P. Enhancing antibacterial activity of chitosan surface by heterogeneous quaternization. Carbohydr. Polym. 2011, 83, 868–875. [Google Scholar] [CrossRef]
- Srinophakun, P.; Thanapimmetha, A.; Plangsri, S.; Vetchayakunchai, S.; Saisriyoot, M. Application of modified chitosan membrane for microbial fuel cell: Roles of proton carrier site and positive charge. J. Clean. Prod. 2017, 142, 1274–1282. [Google Scholar] [CrossRef]
- Prasanth Koppolu, B.; Smith, S.G.; Ravindranathan, S.; Jayanthi, S.; Suresh Kumar, T.K.; Zaharoff, D.A. Controlling chitosan-based encapsulation for protein and vaccine delivery. Biomaterials 2014, 35, 4382–4389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, C.; Li, J.; Kong, M.; Liu, Y.; Cheng, X.J.; Li, Y.; Park, H.J.; Chen, X.G. Surface charge effect on mucoadhesion of chitosan based nanogels for local anti-colorectal cancer drug delivery. Colloids Surf. B Biointerfaces 2015, 128, 439–447. [Google Scholar] [CrossRef]
- Li, B.; Du, L.; Yu, Z.; Sun, B.; Xu, X.; Fan, B.; Guo, R.; Yuan, W.; He, K. Poly (d,l-lactide-co-glycolide) nanoparticle-entrapped vaccine induces a protective immune response against porcine epidemic diarrhea virus infection in piglets. Vaccine 2017, 35, 7010–7017. [Google Scholar] [CrossRef] [PubMed]











| Group | N-2-HACC NPs (%, w/v) | PEDV (mL) | N-2-HACC NPs (g/mL) | Adjuvant Dose (mL) |
|---|---|---|---|---|
| 1 | 0.5 | 1 | 0.01 | 1 |
| 2 | 1 | 1 | 0.02 | 1 |
| 3 | 5 | 1 | 0.1 | 1 |
| 4 | 10 | 1 | 0.2 | 1 |
| C (g/L) | t (s) | ηr | lnηr | lnηr/C | ηsp | ηsp/C |
|---|---|---|---|---|---|---|
| - | 92 | - | - | - | - | - |
| 6 | 474 | 5.1522 | 1.6394 | 0.2732 | 4.1522 | 0.6920 |
| 4 | 364 | 3.9565 | 1.3754 | 0.3438 | 2.9565 | 0.7391 |
| 2.4 | 204 | 2.2174 | 0.7963 | 0.3318 | 1.2174 | 0.5073 |
| 1.71 | 166 | 1.8043 | 0.5902 | 0.3451 | 0.8043 | 0.4704 |
| 1.33 | 147 | 1.5978 | 0.4686 | 0.3524 | 0.5978 | 0.4495 |
| C (g/L) | t (s) | ηr | lnηr | lnηr/C | ηsp | ηsp/C |
|---|---|---|---|---|---|---|
| - | 92 | - | - | - | - | - |
| 5 | 178 | 1.9348 | 0.6600 | 0.1320 | 0.9348 | 0.1870 |
| 3.33 | 146.3 | 1.5092 | 0.4639 | 0.1393 | 0.5902 | 0.1773 |
| 2 | 123 | 13370 | 0.2904 | 0.1452 | 0.3370 | 0.1685 |
| 1.43 | 114 | 1.2391 | 0.2144 | 0.1499 | 0.2391 | 0.1672 |
| 1.1 | 108 | 1.1739 | 0.1603 | 0.1458 | 0.1739 | 0.1581 |
| Sample Solvent | CS | N-2-HACC |
|---|---|---|
| Solubility (mg/mL) | ||
| Normal saline, 0.9% (w/v) | - | 63.9 ± 0.71 |
| Isopropyl alcohol | - | - |
| Deionized water | - | 73 ± 1.41 |
| Acetic acid, 1% (w/v) | 35.1 ± 2.12 | 188.9 ± 4.95 |
| Sodium hydroxide, 1% (w/v) | - | 54.1 ± 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Yu, S.; Qu, W.; Jin, Z.; Zhao, K. Self-Assembly of Soluble Chitosan Derivatives Nanoparticles for Vaccine: Synthesis, Characterization and Evaluation. Polymers 2021, 13, 4097. https://doi.org/10.3390/polym13234097
Liu J, Yu S, Qu W, Jin Z, Zhao K. Self-Assembly of Soluble Chitosan Derivatives Nanoparticles for Vaccine: Synthesis, Characterization and Evaluation. Polymers. 2021; 13(23):4097. https://doi.org/10.3390/polym13234097
Chicago/Turabian StyleLiu, Jinbao, Shuang Yu, Wanying Qu, Zheng Jin, and Kai Zhao. 2021. "Self-Assembly of Soluble Chitosan Derivatives Nanoparticles for Vaccine: Synthesis, Characterization and Evaluation" Polymers 13, no. 23: 4097. https://doi.org/10.3390/polym13234097
APA StyleLiu, J., Yu, S., Qu, W., Jin, Z., & Zhao, K. (2021). Self-Assembly of Soluble Chitosan Derivatives Nanoparticles for Vaccine: Synthesis, Characterization and Evaluation. Polymers, 13(23), 4097. https://doi.org/10.3390/polym13234097