Synthesis and Characterization of Nonwoven Cotton-Reinforced Cellulose Hydrogel for Wound Dressings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
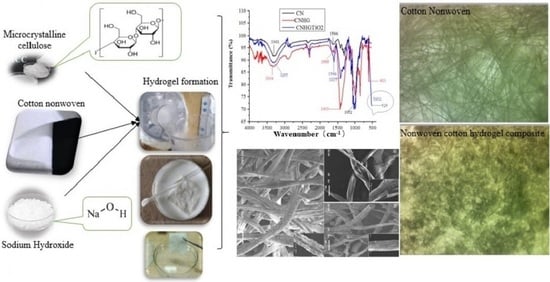
2.2. Nonwoven Cotton Fabric Development
2.3. Development of Cellulose Hydrogel
2.4. Characterization
2.4.1. Scanning Electron Microscopy Analysis
2.4.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.4.3. Mechanical/Comfort Properties Analysis
2.4.4. Exudate-Absorbing Characteristics
2.4.5. Antibacterial Assay
2.4.6. TiO2 Particle Size Analysis
3. Results and Discussion
3.1. Morphology by Scanning Electron Microscopy (SEM)
3.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.3. Air Permeability (AP)
3.4. Moisture Management (MMT)
3.5. Tensile Strength
3.6. Wound Exudate-Absorbing Characteristic Analysis
3.7. Antibacterial Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoekstra, M.J.; Hermans, M.H.; Richters, C.D.; Dutrieux, R.P. A histological comparison of acute inflammatory responses with a hydrofibre or tulle gauze dressing. J. Wound Care 2002, 11, 113–117. [Google Scholar] [CrossRef]
- Boateng, J.; Matthews, K.; Stevens, H.N.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 24–28. [Google Scholar] [CrossRef]
- Skórkowska-Telichowska, K.; Czemplik, M.; Kulma, A.; Szopa, J. The local treatment and available dressings designed for chronic wounds. J. Am. Acad. Dermatol. 2013, 68, 117–126. [Google Scholar] [CrossRef]
- Ghomi, E.R.; Khalili, S.; Khorasani, S.N.; Neisiany, R.E.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef] [Green Version]
- Winter, G.D. Effect of Air Exposure and Occlusion on Experimental Human Skin Wounds. Nat. Cell Biol. 1963, 200, 378–379. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, B.; Wang, J.; Zhang, S.; Lin, Q.; Gong, P.; Ma, L.; Yang, S. A Novel Wound Dressing Based on Ag/Graphene Polymer Hydrogel: Effectively Kill Bacteria and Accelerate Wound Healing. Adv. Funct. Mater. 2014, 24, 3933–3943. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Hayek, S.N.; Gunn, S.W. New technologies for burn wound closure and healing—Review of the literature. Burns 2005, 31, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Sakai, K.; Okano, M. Sustained release (water absorption)—Drug delivery system. Gels Handb. 2001, 2, 46–79. [Google Scholar] [CrossRef]
- Park, K.R.; Nho, Y.C. Synthesis of PVA/PVP hydrogels having two-layer by radiation and their physical properties. Radiat. Phys. Chem. 2003, 67, 361–365. [Google Scholar] [CrossRef]
- Nautiyal, U.; Sahu, N.; Gupta, D. Hydrogel: Preparation, Characterization and Applications. Asian Pac. J. Nurs. Heal. Sci. 2020, 3, 25–32. [Google Scholar] [CrossRef]
- Omidian, H.; Park, K. Introduction to Hydrogels. In Biomedical Applications of Hydrogels Handbook; Springer: New York, NY, USA, 2010; pp. 1–16. [Google Scholar] [CrossRef]
- Bueno, C.Z.; Moraes, Â.M. Development of porous lamellar chitosan-alginate membranes: Effect of different surfactants on biomaterial properties. J. Appl. Polym. Sci. 2011, 122, 624–631. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Jack, E. Lemons Biomaterials Science: An Introduction to Materials in Medicine. Biomater. Sci. 2013, 4–90. [Google Scholar] [CrossRef]
- Neethu, T.; Dubey, P.; Kaswala, A. Prospects and Applications of Hydrogel Technology in Agriculture. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3155–3162. [Google Scholar] [CrossRef]
- Kalhapure, A.; Kumar, R.; Singh, V.P.; Pandey, D.S. Hydrogels: A boon for increasing agricultural productivity in water-stressed environment JSTOR. Curr. Sci. 2016, 111, 1773–1779. [Google Scholar]
- Impact of hydrogel polymer in agricultural sector. Adv. Agric. Environ. Sci. Open Access (AAEOA) 2018, 1, 59–64. [CrossRef]
- Shewan, H.; Stokes, J. Review of techniques to manufacture micro-hydrogel particles for the food industry and their applications. J. Food Eng. 2013, 119, 781–792. [Google Scholar] [CrossRef]
- Batista, R.A.; Espitia, P.J.P.; Quintans, J.D.S.S.; Freitas, M.M.; Cerqueira, M.; Teixeira, J.A.; Cardoso, J.C. Hydrogel as an alternative structure for food packaging systems. Carbohydr. Polym. 2019, 205, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhang, F.; Yuan, R. Applications of natural polymer-based hydrogels in the food industry. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 357–410. [Google Scholar] [CrossRef]
- Parente, M.E.; Andrade, A.O.; Ares, G.; Russo, F.; Jiménez-Kairuz, Á. Bioadhesive hydrogels for cosmetic applications. Int. J. Cosmet. Sci. 2015, 37, 511–518. [Google Scholar] [CrossRef]
- Mitura, S.; Sionkowska, A.; Jaiswal, A.K. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 31–50. [Google Scholar] [CrossRef]
- Aswathy, S.; Narendrakumar, U.; Manjubala, I. Commercial hydrogels for biomedical applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef]
- Kokkarachedu, V.; Gownolla, M.R.; Tippabattini, J.; Murali, M.Y.R.S. A Mini Review on Hydrogels Classification and Recent Developments in Miscellaneous Applications; Elsevier: Amsterdam, The Netherlands, 2017; Volume 79, pp. 958–971. [Google Scholar]
- Sefat, F.; Raja, T.I.; Zafar, M.S.; Khurshid, Z.; Najeeb, S.; Zohaib, S.; Ahmadi, E.D.; Rahmati, M.; Mozafari, M. Nanoengineered Biomaterials for Cartilage Repair; Elsevier: Amsterdam, The Netherlands, 2018; pp. 39–71. [Google Scholar]
- Hunt, J.A.; Chen, R.; Van Veen, T.; Bryan, N. Hydrogels for tissue engineering and regenerative medicine. J. Mater. Chem. B 2014, 2, 5319–5338. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Tavakoli, J.; Mirzaei, S.; Tang, Y. Cost-Effective Double-Layer Hydrogel Composites for Wound Dressing Applications. Polymers 2018, 10, 305. [Google Scholar] [CrossRef] [Green Version]
- Kokabi, M.; Sirousazar, M.; Hassan, Z.M. PVA–clay nanocomposite hydrogels for wound dressing. Eur. Polym. J. 2007, 43, 773–781. [Google Scholar] [CrossRef]
- Queen, D.; Orsted, H.; Sanada, H.; Sussman, G. A dressing history. Int. Wound J. 2004, 1, 59–77. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymers 2008, 49, 5603–5621. [Google Scholar] [CrossRef] [Green Version]
- Hindi, S.; Hindi, S.S.Z. Microcrystalline cellulose: Its processing and pharmaceutical specifications. BioCrystals J. 2016, 1, 26–38. [Google Scholar]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose Aerogels: Synthesis, Applications, and Prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef] [Green Version]
- Núñez-Carmona, E.; Bertuna, A.; Abbatangelo, M.; Sberveglieri, V.; Comini, E.; Sberveglieri, G. BC-MOS: The novel bacterial cellulose based MOS gas sensors. Mater. Lett. 2019, 237, 69–71. [Google Scholar] [CrossRef]
- Lyothibasu, J.P.; Wang, R.H.; Ong, K.; Ong, H.L.; Lee, R.H. Cellulose/carbon nanotube/MnO2 composite electrodes with high mass loadings for symmetric supercapacitors. Cellulose 2021, 28, 3549–3567. [Google Scholar] [CrossRef]
- Oprea, M.; Panaitescu, D.M. Nanocellulose Hybrids with Metal Oxides Nanoparticles for Biomedical Applications. Molecules 2020, 25, 4045. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Alavi, M. Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications. E-Polymers 2019, 19, 103–119. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Colloids Surf. B Biointerfaces 2010, 79, 5–18. [Google Scholar] [CrossRef]
- Prakash, J.; Venkataprasanna, K.; Bharath, G.; Banat, F.; Niranjan, R.; Venkatasubbu, G.D. In-vitro evaluation of electrospun cellulose acetate nanofiber containing Graphene oxide/TiO2/Curcumin for wound healing application. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127166. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Yang, Y.; Fan, A.; Chi, R.; Shi, J.; Zhang, X. Poly (vinyl alcohol) (PVA) hydrogel incorporated with Ag/TiO2 for rapid sterilization by photoinspired radical oxygen species and promotion of wound healing. Appl. Surf. Sci. 2019, 494, 708–720. [Google Scholar] [CrossRef]
- Kubota, Y.; Shuin, T.; Kawasaki, C.; Hosaka, M.; Kitamura, H.; Cai, R.; Sakai, H.; Hashimoto, K.; Fujishima, A. Photokilling of T-24 human bladder cancer cells with titanium dioxide. Br. J. Cancer 1994, 70, 1107–1111. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, Š.; Kralj-Iglič, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M.; Kuo-Kang Liu, I. Mechanical Characterisation of Hydrogels for Tissue Engineering Applications. Top. Tissue Eng. 2008, 4, 1–16. [Google Scholar]
- Ahmad, F.; Mushtaq, B.; Butt, F.A.; Rasheed, A.; Ahmad, S. Preparation and characterization of wool fiber reinforced nonwoven alginate hydrogel for wound dressing. Cellulose 2021, 28, 7941–7951. [Google Scholar] [CrossRef]
- Purdy, A.T. Developments in non-woven fabrics. J. Text. Inst. Proc. 2009, 54, 52–74. [Google Scholar] [CrossRef]
- Yang, J.M.; Lin, H.T. Properties of chitosan containing PP-g-AA-g-NIPAAm bigraft nonwoven fabric for wound dressing. J. Membr. Sci. 2004, 243, 1–7. [Google Scholar] [CrossRef]
- Fang, D.D. Cotton Fiber: Physics, Chemistry and Biology; Springer International Publishing: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Gordon, S.; Hsieh, Y.-L. Cotton: Science and Technology; Woodhead Publishing: Sawston, UK, 2006. [Google Scholar]
- Flax, H.J. The use of cotton sutures in lower abdominal surgery. Surgery 1945, 18, 653–659. [Google Scholar] [CrossRef]
- León, A.; Reuquen, P.; Garín, C.; Segura, R.; Vargas, P.; Zapata, P.; Orihuela, P.A. FTIR and Raman Characterization of TiO2 Nanoparticles Coated with Polyethylene Glycol as Carrier for 2-Methoxyestradiol. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Humphreys, H.; Becker, K.; Dohmen, P.; Petrosillo, N.; Spencer, M.; van Rijen, M.; Wechsler-Fördös, A.; Pujol, M.; Dubouix, A.; Garau, J. Staphylococcus aureus and surgical site infections: Benefits of screening and decolonization before surgery. J. Hosp. Infect. 2016, 94, 295–304. [Google Scholar] [CrossRef]
- Brandes, R.; de Souza, L.; Vargas, V.; Oliveira, E.; Mikowski, A.; Carminatti, C.; Al-Qureshi, H.; Recouvreux, D. Preparation and Characterization of Bacterial Cellulose/TiO2 Hydrogel Nanocomposite. J. Nano Res. 2016, 43, 73–80. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Li, N.; Xia, J.; Meng, Q.; Ding, J.; Lu, J. Synthesis and characterization of TiO2/graphene oxide nanocomposites for photoreduction of heavy metal ions in reverse osmosis concentrate. RSC Adv. 2018, 8, 34241–34251. [Google Scholar] [CrossRef] [Green Version]
- de Dicastillo, C.L.; Correa, M.G.; Martínez, F.B.; Streitt, C.; Galotto, M.J. Antimicrobial Effect of Titanium Dioxide Nanoparticles; Antimicrob. Eff. Titan. Dioxide Nanoparticles, IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Verdier, T.; Coutand, M.; Bertron, A.; Roques, C. Antibacterial Activity of TiO2 Photocatalyst Alone or in Coatings on E. coli: The Influence of Methodological Aspects. Coatings 2014, 4, 670–686. [Google Scholar] [CrossRef]
- Zhu, G.; Kremenakova, D.; Wang, Y.; Militky, J. Air permeability of polyester nonwoven fabrics. Autex Res. J. 2015, 15, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Türkoğlu, G.C.; Sarıışık, A.M.; Karavana, S.Y. Development of textile-based sodium alginate and chitosan hydrogel dressings. Int. J. Polym. Mater. 2020, 70, 916–925. [Google Scholar] [CrossRef]
- Dhineshbabu, N.; Arunmetha, S.; Manivasakan, P.; Karunakaran, G.; Rajendran, V. Enhanced functional properties of cotton fabrics using TiO2/SiO2 nanocomposites. J. Ind. Text. 2016, 45, 674–692. [Google Scholar] [CrossRef]
- Kim, I.; Yoo, M.; Seo, J.; Park, S.; Na, H.; Lee, H.; Kim, S.-K.; Cho, C. Evaluation of semi-interpenetrating polymer networks composed of chitosan and poloxamer for wound dressing application. Int. J. Pharm. 2007, 341, 35–43. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Chang, J.-J.; Yang, M.-C.; Chien, C.-T.; Lai, W.-F. Acceleration of wound healing in diabetic rats by layered hydrogel dressing. Carbohydr. Polym. 2012, 88, 809–819. [Google Scholar] [CrossRef]
- Liu, L.-F.; John, B.; Yeung, K.L.; Si, G. Non-UV based germicidal activity of metal-doped TiO2 coating on solid surfaces. J. Environ. Sci. 2007, 19, 745–750. [Google Scholar] [CrossRef]
- Gogniat, G.; Thyssen, M.; Denis, M.; Pulgarin, C.; Dukan, S. The bactericidal effect of TiO2 photocatalysis involves adsorption onto catalyst and the loss of membrane integrity. FEMS Microbiol. Lett. 2006, 258, 18–24. [Google Scholar] [CrossRef] [Green Version]











| Sample ID | Wetting Time Top (sec) | Wetting Time Bottom (sec) | Top Absorption Rate (%/sec) | Bottom Absorption Rate (%/sec) | Top Max Wetted Radius (mm) | Bottom Max Wetted Radius (mm) | Top Spreading Speed (mm/sec) | Bottom Spreading Speed (mm/sec) |
|---|---|---|---|---|---|---|---|---|
| CN | 9.156 | 119.95 | 49.39 | 0.0 | 10 | 0.0 | 0.684 | 0.0 |
| SD | 3.2 | 2.8 | 2.99 | 3.8 | 2.56 | 2.1 | 2.21 | 2.5 |
| CNHG | 2.766 | 23.166 | 81.21 | 3.75 | 15 | 0.0 | 4.653 | 0.0 |
| SD | 3.5 | 3.33 | 2.98 | 2.89 | 2.51 | 3.4 | 3.33 | 3.4 |
| CNHGTiO2 | 2.203 | 4.43 | 55.016 | 18.51 | 15 | 30 | 6.40 | 5.44 |
| SD | 2.11 | 2.22 | 3.1 | 3.3 | 2.5 | 2.1 | 2.6 | 3.3 |
| Time (hrs.) | CN Wt. (mg) | CNHG Wt. (mg) | CNHGTiO2 Wt. (mg) | Fluid Absorbency % Age (CN) | Fluid Absorbency % Age (CNHG) | Fluid Absorbency % Age (CNHGTiO2) |
|---|---|---|---|---|---|---|
| 0 | 100 | 211 | 215 | 180 | 296 | 314 |
| 2 | 279.7 | 835 | 890 | 180 | 296 | 314 |
| 4 | 300 | 867 | 1006 | 200 | 311 | 368 |
| 6 | 329.2 | 932 | 1038 | 229 | 342 | 383 |
| 8 | 340 | 938 | 1082 | 240 | 345 | 403 |
| 10 | 342.5 | 944 | 1104 | 243 | 347 | 413 |
| 12 | 355.1 | 957 | 1127 | 255 | 354 | 424 |
| 14 | 388.9 | 958 | 1208 | 289 | 354 | 462 |
| 16 | 391.5 | 990 | 1230 | 292 | 369 | 472 |
| 18 | 427 | 1033 | 1235 | 327 | 390 | 474 |
| 20 | 455 | 1101 | 1261 | 355 | 422 | 487 |
| 22 | 480 | 1162 | 1266 | 380 | 451 | 489 |
| 24 | 510 | 1350 | 1390 | 410 | 540 | 547 |
| Average | 361.45 | 944.46 | 1080.92 | 275.38 | 370.54 | 426.92 |
| SD | 104.70 | 258.63 | 291.65 | 74.88 | 67.97 | 70.14 |
| CV% | 28.97 | 27.38 | 26.98 | 27.19 | 18.34 | 16.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, F.; Mushtaq, B.; Butt, F.A.; Zafar, M.S.; Ahmad, S.; Afzal, A.; Nawab, Y.; Rasheed, A.; Ulker, Z. Synthesis and Characterization of Nonwoven Cotton-Reinforced Cellulose Hydrogel for Wound Dressings. Polymers 2021, 13, 4098. https://doi.org/10.3390/polym13234098
Ahmad F, Mushtaq B, Butt FA, Zafar MS, Ahmad S, Afzal A, Nawab Y, Rasheed A, Ulker Z. Synthesis and Characterization of Nonwoven Cotton-Reinforced Cellulose Hydrogel for Wound Dressings. Polymers. 2021; 13(23):4098. https://doi.org/10.3390/polym13234098
Chicago/Turabian StyleAhmad, Faheem, Bushra Mushtaq, Faaz Ahmed Butt, Muhammad Sohail Zafar, Sheraz Ahmad, Ali Afzal, Yasir Nawab, Abher Rasheed, and Zeynep Ulker. 2021. "Synthesis and Characterization of Nonwoven Cotton-Reinforced Cellulose Hydrogel for Wound Dressings" Polymers 13, no. 23: 4098. https://doi.org/10.3390/polym13234098
APA StyleAhmad, F., Mushtaq, B., Butt, F. A., Zafar, M. S., Ahmad, S., Afzal, A., Nawab, Y., Rasheed, A., & Ulker, Z. (2021). Synthesis and Characterization of Nonwoven Cotton-Reinforced Cellulose Hydrogel for Wound Dressings. Polymers, 13(23), 4098. https://doi.org/10.3390/polym13234098